2010-1999 Publications

Peinador, C.; Pia, E.; Blanco, V.; Garcia, M. D.; Quintela, J. M., Complexation of Pyrene in Aqueous Solution with a Self-Assembled Palladium Metallocycle. Org. Lett. 2010, 12, 1380-1383. DOI: 10.1021/ol1004577
A bidentate ligand based on N-monoaryl 4,4'-bipyridinium undergoes self-assembly to dinuclear rectangular metallocycles upon coordination to palladium(II) and platinum(II) centers. These metallocycles form a very stable complex with pyrene in aqueous solution and in the solid state. A crystal structure of the pyrene Inclusion complex is presented. The association constants between pyrene and metallocycle 3a in organic solvents and water (K-a = 2.3 x 10(6)) were determined.
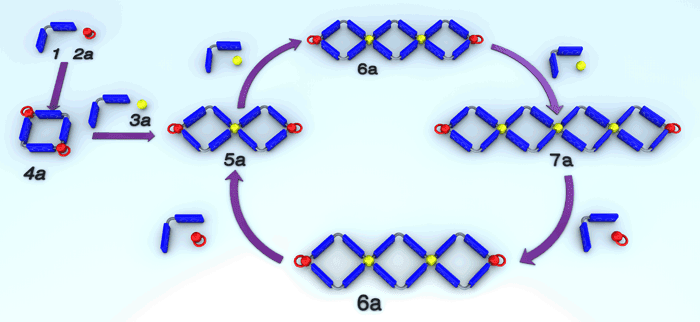
Blanco, V.; Garcia, M. D.; Platas-Iglesias, C.; Peinador, C.; Quintela, J. M., Dynamic formation of self-organized corner-connected square metallocycles by stoichiometric control. Chem. Commun. 2010, 46, 6672-6674. DOI: 10.1039/c0cc01841d
Corner-connected molecular squares were self-assembled from a three-component system formed by a bidentate ligand and two palladium or platinum complexes. The system is dynamic and the constitution of the species in solution can be modulated under stoichiometric control.

Blanco, V.; Garcia, M. D.; Terenzi, A.; Pia, E.; Fernandez-Mato, A.; Peinador, C.; Quintela, J. M., Complexation and Extraction of PAHs to the Aqueous Phase with a Dinuclear PtII Diazapyrenium-Based Metallacycle. Chem. Eur. J. 2010, 16, 12373-12380. DOI: 10.1002/chem.201002051
New palladium and platinum metallacycles have been synthesized by reaction between a 2,7-diazapyrenium-based ligand and Pd-II and Pt-II complexes. The inclusion complexes between the metallacycles and polycyclic aromatic hydrocarbons (PAHs) in CD3NO2 and D2O were studied by NMR spectroscopy. The structures of the inclusion complexes of the Pt metallacycle as host with pyrene, phenanthrene, and triphenylene were confirmed by single crystal X-ray crystallography. The association constants between the Pt metallacycle and the selected PAHs were determined in CH3CN following the characteristic charge-transfer band displayed in their UV/Vis absorption spectrum. Although in aqueous solution all the complexes showed a 1:1 stoichiometry, in CH3CN the Job plot indicated a 2:1 stoichiometry for complexes with triphenylene and benzo[a]pyrene. The estimated association constants in water correlate with the hydrophobicity of the PAH, indicating that hydrophobic forces play an important role in the complexation process.
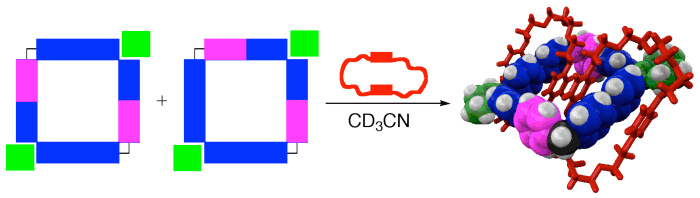
Blanco, V.; Abella, D.; Pia, E.; Platas-Iglesias, C.; Peinador, C.; Quintela, J. M., Regioselective Catenation of Dinuclear Palladium and Platinum Metallocycles Promoted by π-π Interactions. Inorg. Chem. 2009, 48, 4098-4107. DOI: 10.1021/Ic8022425
Dinuclear metallocycles were assembled from an L-shaped bidentate ligand based on 4,4'-bipyridine and palladium or platinum square-planar cis complexes. The palladium (4a,b) or platinum (5a,b) square metallocycles were obtained as 1:1 regioisomeric mixtures depending on which pair of coordinative nitrogen atoms binds to the metal center. The squares display a pi-deficient cavity suitable to incorporate two pi-donor aromatic systems. Therefore, the reaction with macrocyclic polyethers (BPP34C10 or DN38C10) resulted in the regioselective self-assembly of the [3]catenanes 4a(BPP34C10 or DN38C10)(2)center dot 6PF(6) and 5a(BPP34C10 or DN38C10)(2)center dot 6PF(6) because only macrocycles 4a and 5a present the correct disposition of the pi-deficient aromatic systems to maximize the;pi center dot center dot center dot pi stacking interactions. Single-crystal X-ray analyses of [3]catenanes revealed that the structures are additionally stabilized by [C-H center dot center dot center dot O], [N-H center dot center dot center dot O] bonds and [C-H center dot center dot center dot pi] interactions. The 1:2 inclusion complexes of metallocycles were prepared by self-assembly of three components: the ligand 1-(4-(pyridin-4-yl)benzyl)-4,4'-bipyridin-1-ium, a square planar complex M(en)(NO3)(2) (M = Pd or Pt, en = ethylenediamine), and a dioxoaromatic guest in a 2:2:2 ratio. The comparative study of the formation of 1:2 inclusion complexes has allowed us to conclude that the pi center dot center dot center dot pi interactions between the host and guests are responsible for the observed regioselectivity.
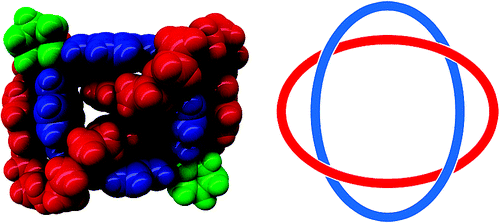
Peinador, C.; Blanco, V.; Quintela, J. M., A New Doubly Interlocked [2]Catenane. J. Am. Chem. Soc. 2009, 131, 920-921. DOI: 10.1021/Ja8088372
The synthesis and the crystal structure of a doubly braided [2]catenane, a new molecular Solomon link, obtained by a 5-component-self-assembly process based on coordinative bonds, pi-donor/pi-acceptor interactions, and hydrogen bonding is reported.
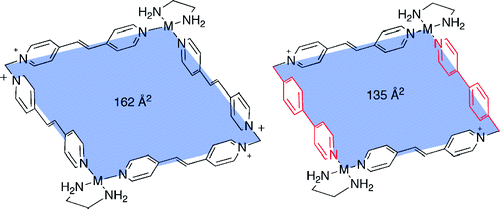
Blanco, V.; Gutierrez, A.; Platas-Iglesias, C.; Peinador, C.; Quintela, J. M., Expanding the Cavity Size: Preparation of 2:1 Inclusion Complexes Based on Dinuclear Square Metallocycles. J. Org. Chem. 2009, 74, 6577-6583. DOI: 10.1021/Jo901034c
The self-assembly of two new ligands based on the trans-1,2-bis(4-pyridyl)ethylene motif with palladium or platinum complexes led to quadrangular metallocycles. 1,1'-Methylenebis(4-((E)-2-(pyridin-4-yl)vinyl)pyridinium gave the square metallocycles, while the second ligand, which is less symmetrical, gave a mixture of the regioisomeric metallocyles. The metallocycles display the ideal disposition of the pi-acceptor units to maximize the pi-stacking interactions with aromatic guests. Thus, molecular recognition of pi-donor aromatic guests by square metallocycles produced the corresponding 2:1 inclusion complexes. On the otherhand, starting from the mixture of regioisomers, the incorrect regioisomer rearranged to the correct metallocycle upon the addition of aromatic guests. On the basis of this behavior, a [3]catenane was obtained regioselectively from the mixture of the regioisomeric metallocycles and the appropriate cyclophane. The formation of this catenane was confirmed by NMR and X-ray crystallographic studies.
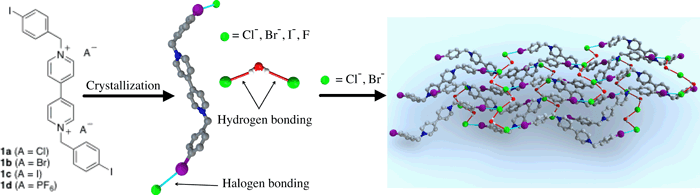
Garcia, M. D.; Blanco, V.; Platas-Iglesias, C.; Peinador, C.; Quintela, J. M., Interplay between Halogen/Hydrogen Bonding and Electrostatic Interactions in 1,1'-Bis(4-iodobenzyl)-4,4'-bipyridine-1,1'-diium Salts. Cryst. Growth Des. 2009, 9, 5009-5013. DOI: 10.1021/Cg901175e
We present herein the crystal structures I-IV of a series of 1,1'-bis(4-iodobenzyl)-4,4'-bipyridine-1,1'-diium salts 1a-d. The inherent structural features of these viologen derivatives, i.e. the electrostatic potential distribution oil the dication 1(2+) and the nature of the counteranions, give rise to a series of potential hydrogen/halogen bond synthons A-E that, in conjunction with electrostatic interactions, play a key role in the supramolecular organization of these salts in the crystalline state. A subtle combination of hydrogen and halogen bonding guides the solid state assembly of hydrates I-II, leading to 2D -> 2D parallel 3-fold interpenetration of (6,3) noncovalent nets.
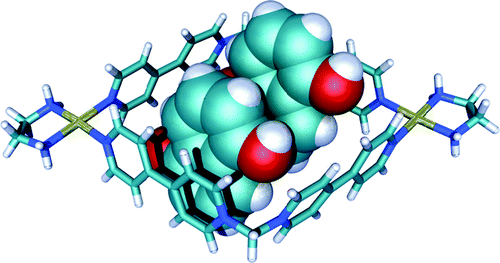
Blanco, V.; Chas, M.; Abella, D.; Pia, E.; Platas-Iglesias, C.; Peinador, C.; Quintela, J. M., Self-assembly of 1:2 inclusion complexes between a metallocycle host and dihydroxyaromatic guests: A redox controlled complexation process. Org. Lett. 2008, 10, 409-412. DOI: 10.1021/Ol702701t
Two new 1:2 inclusion complexes were prepared by self-assembly of three components: a ligand based on 4,4'-bipyridinium, a square-planar Pd complex, and a dihydroxyaromatic guest (hydroquinone or 1,5-dihydroxynaphthalene) in a 2:2:2 ratio. Their X-ray structural analyses revealed that the complexes are stabilized by pi-pi stacking and [C-H center dot center dot center dot pi] interactions.
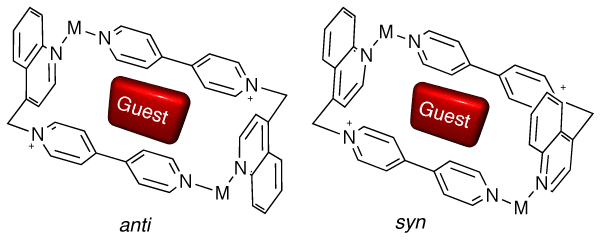
Abella, D.; Blanco, V.; Pia, E.; Chas, M.; Platas-Iglesias, C.; Peinador, C.; Quintela, J. M., Stereoselective self-assembly of atropoisomeric Pd(II) metallocycles induced by an aromatic guest. Chem. Commun. 2008, 2879-2881. DOI: 10.1039/B802213e
A new dinuclear Pd(II) metallocycle consisting of a 4,4'- bipyridin-1- ium ligand with a quinoline moiety was self-assembled in aqueous solution with the aid of template molecules; the situation found in solution in which both syn and anti or only the anti atropoisomers are observed strongly relies on the intermolecular host-guest interactions.
Fernández-Mato, A.; Blanco, G.; Quintela, J. M.; Peinador, C., Synthesis of new bis(2-[1,8]naphthyridinyl) bridging ligands with multidentate binding sites. Tetrahedron 2008, 64, 3446-3456. DOI: 10.1016/j.tet.2008.02.017
A series of new 2-[1,8]naphthyridinyl and bis(2-[1,8]naphthyridinyl) bridging ligands with multidentate binding sites were prepared using 2-amino-5-cyano-6-ethoxy-4-phenyl-3-carbaldehyde pyridine as excellent Friedl‰nder synthon. Condensation with a series of acetyl(heteroaryl)aromatics provides the corresponding 2-aryl(heteroaryl)-1,8-naphthyridines. Reaction with 1,3-diacetylbenzene, 2,6-diacetylpyridine or 4-tert-butyl-2,6-diacetylpyridine provides the expected Friedl‰nder product. Similar 2:1 condensation with 1,4-diacetylbenzene, 4,4'-diacetylbiphenyl, 1,4- and 1,6-diacetylpyrene, 2,6-diacetylpyrazine or 2,3-butanedione leads to a family of six new bis-1,8-nap ligands. The reaction with cyclic 1,2- or 1,3-diketones affords 3,3'-annelated derivatives of all-syn or all-trans planar 2,2'-nap, respectively. Examination of the electronic absorption and emission spectra of the bridging ligands was realized.
Blanco, G.; Quintela, J. M.; Peinador, C., Application of the aza-Wittig reaction to the synthesis of pyrazinothienotriazolopyrimidinones: a new tetracyclic ring system. Tetrahedron 2008, 64, 1333-1344. DOI: 10.1016/J.Tet.2007.11.059
A simple one-pot and efficient method is described for the synthesis of pyrazinothienopyrimidines 6 by domino processes involving aza-Wittig/intermolecular nucleophilic addition/intramolecular cyclization. A tandem aza-Wittig reaction of phosphazenes 7, derived from 6, with heterocumulenes (isocyanates, carbon disulfide or carbon dioxide) generates the pyrazinothienotriazolopyrimidinones 9, 11 and 12, respectively. Pyrazino[2',3':4,5]thieno[3,2-d]-1,2,4-triazolo[1,5-a]pyrimidin-4(3H)-ones 15 and bis(pyrazinothienotriazolopyrimidinones) 17 were synthesized by the intermolecular aza-Wittig reaction of phosphazenes 7 with acyl chlorides or alpha,omega-dichlorides followed by heterocyclization via imidoyl chloride intermediate 16. Further S-alkylation of 11 and reaction of 6 with phosgeniminium chloride produce 2-alkylthio- and 2-N,N-dimethylaminopyrazinothienotriazolopyrimidinones 13 and 19, respectively.
Blanco, G.; Quintela, J. M.; Peinador, C., Synthesis of pyrazinothienopyrimidine derivatives by the application of the intramolecular and intermolecular aza-Wittig reaction/heterocyclization. Synthesis 2008, 1397-1403. DOI: 10.1055/S-2008-1072514
Pyrazino[2',3':4,5]thieno[3,2-d]pyrimidine derivatives 6 were synthesized by the intermolecular aza-Wittig reaction of N-heteroaryl phosphazene derivatives with carboxylic acid chlorides. The heterocyclization occurs via imidoyl chloride intermediates derived from phosphazenes 4a,b, obtained in a one-step process from alpha-azidothieno[2,3-b]pyrazine carboxylic acid 3. Moreover, cyclic pyrazinothienopyrimidines were prepared, in a two-step procedure, from amides and azidothienopyrazine-alpha-carbonyl chloride using an intramolecular aza-Wittig heterocyclization reaction strategy.
Blanco, G.; Fernandez-Mato, A.; Quintela, J. M.; Peinador, C., An efficient one-pot three-step domino synthesis of substituted bis(pyrazino[2',3':4,5]thieno[3,2-d]pyrimidin-4(3H)-ones). Tetrahedron 2008, 64, 11136-11143. DOI: 10.1016/J.Tet.2008.09.067
A series of bis(pyridinium)-xylylene derivatives bearing carboxylate terminal groups were investigated as guests for the cucurbit[7]uril host in aqueous solution. While the presence of the terminal carboxylates has a modest effect on the thermodynamic stability of the complexes, the kinetics of complex association/dissociation is strongly affected. The relative position (meta, para) of the carboxylate group in relation to the pyridinium nitrogen also exerts a considerable effect on the binding kinetics.
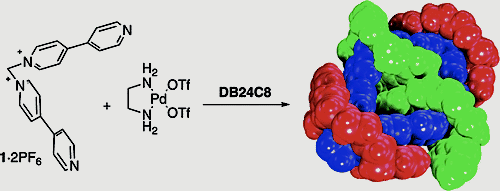
Blanco, V.; Chas, M.; Abella, D.; Peinador, C.; Quintela, J. M., Molecular catenation via metal-directed self-assembly and π-donor/π-acceptor interactions: Efficient one-pot synthesis, characterization, and crystal structures of [3]catenanes based on Pd or Pt dinuclear metallocycles. J. Am. Chem. Soc. 2007, 129, 13978-13986. DOI: 10.1021/Ja074721a
Dinuclear square metallocycles 3a,b assemble spontaneously when M(en)(OTf)(2) (M = Pd, Pt) and a 4,4'-bipyridinium ligand are mixed in acetonitrile. Six new [3]catenanes were prepared in good yields by thermodynamically driven self-assembly reaction of molecular squares 3a,b and pi-complementary dioxoaryl cyclophanes. Single-crystal X-ray analyses of the [3]catenanes revealed the insertion of two aromatic units inside the metallocycle cavity. The structures are stabilized by means of a combination of pi-pi stacking, [C-H center dot center dot center dot pi] interactions, and [C-H center dot center dot center dot O] hydrogen bonds. [3]Catenane (DB24C8)(2)-(3a) showed in solid-state two external DB24C8 rings positioned over the Pd(en) corners, which are held in position by [N-H center dot center dot center dot O] hydrogen bonds. Furthermore, formation of catenane (DB24C8)(2)-(3a) can be switched off and on in a controllable manner by successive addition of KPF6 and 18-crown-6.
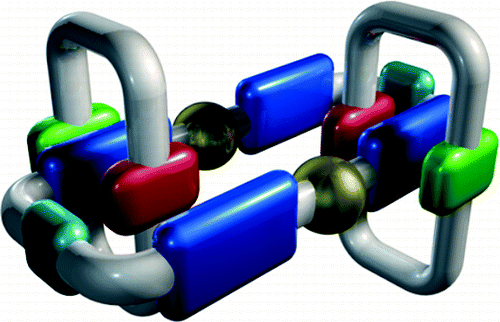
Chas, M.; Blanco, V.; Peinador, C.; Quintela, J. M., Synthesis of [3]catenanes based on metal-directed self-assembly and π-donor/π-acceptor interactions. Org. Lett. 2007, 9, 675-678.. DOI: 10.1021/Ol062824c
A metal-directed self-assembly of [3]catenanes in combination with pi-pi interactions was investigated. Ligands based on 4,4'-bipyridinium or 2,7-diazapyrenium were used in conjunction with dioxoaryl cyclophanes (4-6) and trans-PdCl2(CH3CN)(2). The [3]catenanes show a dinuclear palladium 46-membered metallomacrocycle interlocked by two pi-complementary dioxoaryl macrocycles.
Chas, M.; Abella, D.; Blanco, V.; Pia, E.; Blanco, G.; Fernandez, A.; Platas-Iglesias, C.; Peinador, C.; Quintela, J. M., Synthesis of new self-assembled PdII and PtII rectangular metallomacrocycles: A comparative study of their inclusion complexes. Chem. Eur. J. 2007, 13, 8572-8582. DOI: 10.1002/Chem.200700575
New palladium and platinum metallocycles have been synthesized by reacting 4,4(')-bipyridinium-based ligands with Pd-II and Pt-II complexes. Strict thermodynamic self-assembly of 1 and [M(en)(NO3)(2)] (M = Pd, Pt) 6a,b afforded metallocycles 7a,b. However, the synthesis of 8a,b and 9a,b required a self-assembly process that used sodium p-phenylenediacetate (12) as a template. Finally, metallocycles 10a,b were synthesized under high dilution conditions from ligand 4. The formation of inclusion complexes between metallocycles 7-10 and substrates 13 and 14 were studied by low-temperature H-1 NMR, and the association constants were determined in nitromethane and water by following the characteristic charge-transfer band that these metallomacrocycles show in their UV-visible absorption spectra. A clear correlation between the affinity for a substrate and the dimensions of the metallocycle was observed. Metallocycles 8b and 9b exhibited the highest binding constants in water and nitromethane. This observation is in agreement with the DFT (B3LYP)-optimized geometries obtained for the different metallomacrocycles, which indicate that only macrocycles 8 and 9 possess a cavity with a width larger than 3.5 angstrom. The insertion of hydroquinone or diol 13 into the cavity of metallocycle 11a was confirmed by single-crystal X-ray crystallography.
Blanco, G.; Quintela, J. M.; Peinador, C., Efficient one-pot preparation of bis(pyrazino[2',3':4,5]thieno-[3,2-d]pyrimidin-4-yl)benzenes based on an aza-Wittig/mediated annulation strategy. Tetrahedron 2007, 63, 2034-2041. DOI: 10.1016/J.Tet.2006.12.049
Aza-Wittig mediated annulation provides a highly facile and straightforward one-pot strategy for the synthesis of bis(pyrazino[2',3':4,5]thieno[3,2-d]pyrimidin-4-yl)benzenes 5 and 7. A tandem aza-Wittig reaction of iminophosphorane 2 with 1,4- or 1,3-phenylene diisocyanate, followed by intramolecular heteroconjugate addition annulation after addition of a nucleophilic reagent (amine, phenol, thiophenol or ROH), in presence of catalytic K2CO3 Or NaOR, gives selectively the functionalized bis(pyrazinothienopyrimidinones) 5 and 7.
1999
E. ROMAN, S. MENDC. PEIOZA, NADOR, A. E. KAIFERImproved Synthesis of Cavitands
J. Org. Chem., 1999, 6477-257, 258. doi: 10.1021/jo982218y
C. PEINADOR, E. ROMÁN, K. ABBOUD, A. E. KAIFER
Novel Cavitands Containing Electrochemically Active 4,4'-bipyridinium Subunits.
Chem. Commum., 1999, 1887-1888. doi: 10.1039/a903164b
J. M. QUINTELA, C. PEINADOR, L. M. GONZÁLEZ, R. RIGUERA, I. RIOJA, M. C. TERENCIO, A. UBEDA, M. J. ALCARAZ
Synthesis and Pharmacological Evaluation of Some 8-Cyanopyrido[3′,2′:4,5]-thieno[3,2-d]triazine Derivatives as Inhibitors of Nitric Oxide and Eicosanoid Biosynthesis
J. Med. Chem., 1999, 42, 4720-4724. doi: 10.1021/jm991085l
2000
A. VIDAL, M. L. FERRÁNDIZ, A. UBEDA, I. GUILLÉN, R. RIGUERA, J. M. QUINTELA, C. PEINADOR, M. J. MOREIRA, M. J. ALCARAZ
Effects of Some Isoxazolpyrimidine Derivatives on Nitric Oxide and Eicosanoid Biosynthesis
Life Sciences, 2000, 66, PL125-PL131. doi: 10.1016/S0024-3205(99)00658-X
J. M. QUINTELA, M. J. MOREIRA, C. PEINADOR
A Convenient Method for the Synthesis of Thiopyrano[2,3-d:6,5-d']dipyrimidine Derivatives
Heterocycles, 2000, 52, 333-348. doi: 10.3987/COM-99-S29
RIOJA, I., UBEDA, M., TERENCIO, M. C, I. GUILLÉN, R. RIGUERA, J. M. QUINTELA, C. PEINADOR, L. M. GONZÁLEZ, M. J. ALCARAZ
An Anti-inflammatory Ditriazine Inhibiting Leukocyte Functions and Expression of Inducible Nitric Oxide Sinthase and Cyclo-oxygenase-2
Eur. J. Pharm., 2000, 397, 207-217. doi:10.1016/S0014-2999(00)00243-0
2001
NAUMANN, C.; ROMÁN, E.; PEINADOR, C.; REN, T.; PATRICK, B. O.; KAIFER, A. E.; SHERMAN, J. C.
Expanding Cavitand Chemistry: The Preparation and Characterization of Cavi[n]tands with n³4
Chem. Eur. J., 2001, 7, 1637-1649. doi: 10.1002/1521-3765(20010417)7:8<1637::AID-CHEM16370>3.0.CO;2-X
R. TOBA, J. M. QUINTELA, C. PEINADOR, E. ROMÁN, A. E. KAIFER
A New Series of Dendrimers With 4,4'-bipyridinium Cores Capable of Fast Electron Transfer Reactions
Chem. Commum., 2001, 857-858. doi: 10.1039/b102134f
R. ALVAREZ-SARANDÉS, C. PEINADOR, J. M. QUINTELA
Iminophosphoranes in Heterocyclic Chemistry. A Simple One-pot Synthesis of Pyridothienopyridazines and Pyrimidothienopyridazines
Tetrahedron, 2001, 57, 5413-5420. doi: 10.1016/S0040-4020(01)00460-4
J. M. QUINTELA, C. PEINADOR, M. J. MOREIRA, A. ALONSO, L. M. BOTANA, R. RIGUERA.
Pyrazolopyrimidines: Synthesis, effect on Histamine release from Rat Peritoneal Mast Cells and Cytotoxic activity
Eur. J. Med. Chem., 2001, 36, 321-332. doi: 10.1016/S0223-5234(01)01225-9
2002
J. M. QUINTELA, C. PEINADOR
Recent Advances in the Synthesis of Thiophene Polycondensed Heterocycles of Biological Activity
Recent Res. Devel. Organic Chem., 2002, 6, 565-585. ISBN: 81-7895-041-3.
E. ROMÁN, M. CHAS, J. M. QUINTELA, C. PEINADOR, A. E. KAIFER
Synthesis and Electrochemical Properties of Cavitands Functionalized with 4,4′-Bipyridinium Units
Tetrahedron, 2002, 58, 699-709. doi: 10.1016/S0040-4020(01)01109-7
J. LIU, W. ONG, A. E. KAIFER, C. PEINADOR
A Macrocyclic Effect on the Formation of Capped Silver Nanoparticles in DMF
Langmuir, 2002, 18, 5981-5983. doi: 10.1021/la025956x
R. TOBA, J. M. QUINTELA, C. PEINADOR, E. ROMÁN, A. E. KAIFER
Dendritic Cavitands: Preparation and Electrochemical Properties
Chem. Commun., 2002, 1768-1769. doi: 10.1039/b204572a
2003
J. M. QUINTELA, C. PEINADOR, L. GONZÁLEZ, I. DEVESA, M. L. FERRANDIZ, M. J. ALCARAZ, R. RIGUERA.
6-Dimethylamino 1H-Pyrazolo[3,4-d]Pyrimidine Derivatives as New Inhibitors of Inflammatory Mediators in Intact Cells
Bioorg. Med. Chem., 2003, 11(6), 863-868. doi: 10.1016/S0968-0896(02)00562-X
J. M. QUINTELA, C. PEINADOR, L. GONZÁLEZ, R. IGLESIAS, A. PARAMA, F. ÁLVAREZ, M. L. SANMARTÍN, R. RIGUERA.
Piperazine N-substitued naphthyridines, pyridothienopyrimidines and pyridothienotriazines: new antiprotozoals active against Philasterides dicentrarchi
Eur. J. Med. Chem., 2003, 38(3), 265-275. doi: 10.1016/S0223-5234(03)00032-1
2004
D. VÁZQUEZ, C. PEINADOR, J. M. QUINTELA
Syntheis of pyrido and pyrazinothienopyrimidine-4,8(3H,9H)-diones derivatives by the aza-Wittig methodology
Tetrahedron, 2004, 60, 275-283. doi: 10.1016/j.tet.2003.11.021
A. PARAMÁ, R. IGLESIAS, F. LEIRO, J. M. QUINTELA, C. PEINADOR, L. GONZÁLEZ, R. RIGUERA, M. L. SANMARTÍN
In Vitro efficacy of new antiprotozoals against Philasterides dicentrarchi (Ciliophora, Scuticociliatida)
Dis. Aquat. Org. 2004, 62, 97-102. doi: 10.3354/dao062097
2005
W. ONG, J. GRINDSTAFF, D. SOBRANSINGH, R. TOBA, J. M. QUINTELA, C. PEINADOR, A. E. KAIFER.
Electrochemical and Guest Binding Properties of FŽchet- and Newkome-Type Dendrimers with a Single Viologen Unit Located at Their Apical Positions
J. Am. Chem. Soc., 2005, 127, 3353-3361. doi: 10.1021/ja049530b
J. VILAR, C. PEINADOR, J. M. QUINTELA.
A Synthetic Route To Pyridazino[4,5-b]-1,8-Naphthyridines, a New Tetraazaheterocyclic System
Heterocycles, 2005, 65 (2), 329-336. >doi: 10.3987/COM-04-10265
Autores: J. M. QUINTELA, C. PEINADOR
Pyridopyrimidines, pyrazolopyrimidines, pyridothienopyrimidines and pyridothienotriazines. Synthesis and biological activity
Trends in Heterocyclic Chemistry, 2005, 10, 97-114 .
2006
E. PÍA, R. TOBA, M. CHAS, C. PEINADOR, J. M. QUINTELA.
Synthesis of new viologen macrocycles with intramolecular charge transfer
Tetrahedron Lett., 2006, 47, 1953-1956. doi:10.1016/j.tetlet.2006.01.073
M. CHAS, C. PLATAS-IGLESIAS, C. PEINADOR, J. M. QUINTELA.
New self-assembled dinuclear Pd(II) and Pt(II) metallomacrocycles of a 4,4′-bipyridin-1-ium ligand with an inner cavity
Tetrahedron Lett., 2006, 47, 3119-3122. doi:10.1016/j.tetlet.2006.02.146
C. VEIGA, J. M. QUINTELA, C. PEINADOR, M. CHAS, A. FERNÁNDEZ.
A Facile Synthesis of Substituted 1,8-Naphthyridines Based on the Aza-Wittig/Electrocyclic Ring Closure Strategy
Heterocycle, 2006, 68 (2), 295-306. doi: 10.3987/COM-05-10629
J. M. QUINTELA, C. PEINADOR, M. J. MOREIRA, R. TOBA, M. CHAS.
A Ready One-Pot Preparation for Pteridine and Isoxazolo[3,4-d]Pyrimidine Derivatives
Heterocycles, 2006, 68, 933-947. doi: 10.3987/COM-06-10686
M. CHAS, E. PÍA, R. TOBA, C. PEINADOR, J. M. QUINTELA
New Hybrid [2]Catenanes Based on a 4,4′-Bipyridinium Ligand
Inorg. Chem., 2006, 45, 6617-6619. doi: 10.1021/ic0605165
G. BLANCO, N. SEGUÍ, J. M. QUINTELA, C. PEINADOR, M. CHAS R. TOBA
A Practical One-Pot Procedure for the Synthesis of Pyrazino[2′,3′:4,5] thieno[3,2-d]pyrimidinones by a Tandem Aza-Wittig/Heterocumulene-Mediated Annulation Strategy
Tetrahedron, 2006, 62, 11124-11135. doi:10.1016/j.tet.2006.09.028
G. BLANCO, J. M. QUINTELA, C. PEINADOR
Synthesis of New Heteroaromatic Nitrogen Ligands: Pyrimido-[4,5:4',5']-thieno[3',2':4,5]thieno[3,2-d]pyrimidines and 1,2,3-Triazine[4,5:4',5']thieno[3',2':4,5]thieno[3,2-d]-1,2,3-triazines
J. Heterocycl. Chem., 2006, 43, 1051-1056. doi:10.1002/jhet.5570430435
J. M. QUINTELA, C. PEINADOR
Pyridothieno-1,2,3-ditriazines, pyridothienotriazines, and isoxazolo and pyrazolopyrimidine derivatives. Synthesis and anti-inflammatory, analgesic and anti-angiogenesis activity
Trends in Heterocyclic Chemistry, 2006, 11, 33-51.