2026-2021 Publications

Blanco-Gómez, A.; Barravecchia, L.; Scarel, E.; De Zorzi, R.; Colomina-Alfaro, L.; Bandiera, A.; Kralj, S.; Vila, A.; Porrelli, D.; Peinador, C.; García, M. D.; Marchesan, S. Dual pH-Responsive Pseudopeptide: Hydrogelation and Self-Assembly into Single-and Multi-Walled Nanotubes. Nanoscale 2026, , accepted. DOI: 10.1039/D5NR04076K
This work reports the heterochiral tripeptide L-Phe-D-Phe-L-Phe, N-capped with vermellogen, as an ionizable pseudopeptide hydrogelator, and how the pH-responsiveness is transferred from the molecular to the nanoscale, and all the way up to the macroscale through self-assembly. In particular, the protonation of the vermellogen moiety is responsible for hydrogelation, while that of the peptide fine-tunes the matrix nanostructure and viscoelastic properties. Electron microscopy reveals the correlation of varying viscoelastic properties with the nanostructure of the hydrogel matrices. Self-assembly of the pseudopeptide undergoes a peculiar evolution from nanofibers to nanotubes. This process depends on the degree of Cterminal deprotonation, notably with the gradual increase in the internal diameter of the resulting nanotubes as the deprotonation progresses to completion. State-of-the-art characterization techniques confirm that the nanostructured gels are predominantly comprised of parallel β-sheets as primary self-assembling motifs, arranging vermillogen units in a clockwise helical pattern, which minimizes electrostatic repulsions. The evolution from nanofibers to nanotubes appears to be driven by a long-range hydrogen bonding interaction, involving the hydrazone group and the deprotonated C-terminus of adjacent β-sheets. The potential biomedical application of the gels is demonstrated through the controlled release of a model anticancer drug, and in vitro cytocompatibility assays.
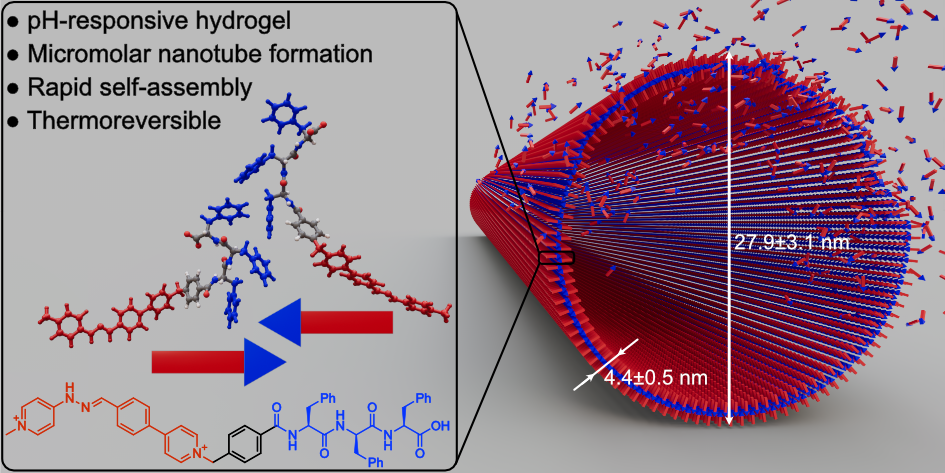
Vila, A.; Gauci, V.; Blanco-Gómez, A. Highly stable self-assembled nanotubes from a bipyridinium-based amphiphilic pseudopeptide. Chem. Commun. 2026, , accepted. DOI: 10.1039/D5CC06185G
Herein, we report an amphiphilic pseudopeptide that spontaneously self-assembles into monodisperse cationic nanotubes under physiological conditions. These nanotubes exhibit fast self-assembly, remarkable thermal stability and thermoreversibility. The self-assembly concentration range is exceptionally broad, enabling the formation of stable hydrogels at concentrations above 5 mM while preserving the nanostructure at micromolar levels.
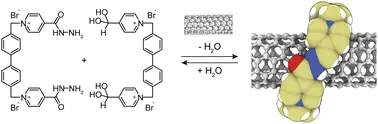
Villalva, J.; Blanco-Gómez, A.; Jiménez, D. M.; López-Moreno, A.; Ruiz-González, M. L.; Peinador, C.; García, M. D.; Pérez, E. M. Extended white-box cyclophanes for the synthesis of mechanically interlocked derivatives of single-walled carbon nanotubes in water. Chem. Sci. 2025, , accepted. DOI: 10.1039/D5SC05224F
Single-walled carbon nanotubes (SWNTs) possess exceptional properties, but their inherent tendency to agglomerate has limited their exploitation. Here, we present a strategy for the aqueous synthesis of mechanically interlocked nanotube derivatives (MINTs) by combining two complementary cationic molecules that not only assist in dispersing SWNTs but also assemble around them through dynamic acyl hydrazone linkages. The resulting MINTs integrate the stability of covalent modification with the unique versatility of acyl hydrazone functionalities, enabling post-functionalization of the nanotube surface. Comprehensive characterization confirmed the successful formation of these interlocked structures, accompanied by smaller fractions of other supramolecular aggregates, while preserving the SWNT integrity. Importantly, the acyl hydrazone moieties impart intrinsic hydrolytic susceptibility, facilitating the controlled recovery of pristine nanotubes after use. This waterborne MINT platform offers a promising route for developing functional SWNT materials tailored for applications requiring both stability and reversible modification in aqueous environments.
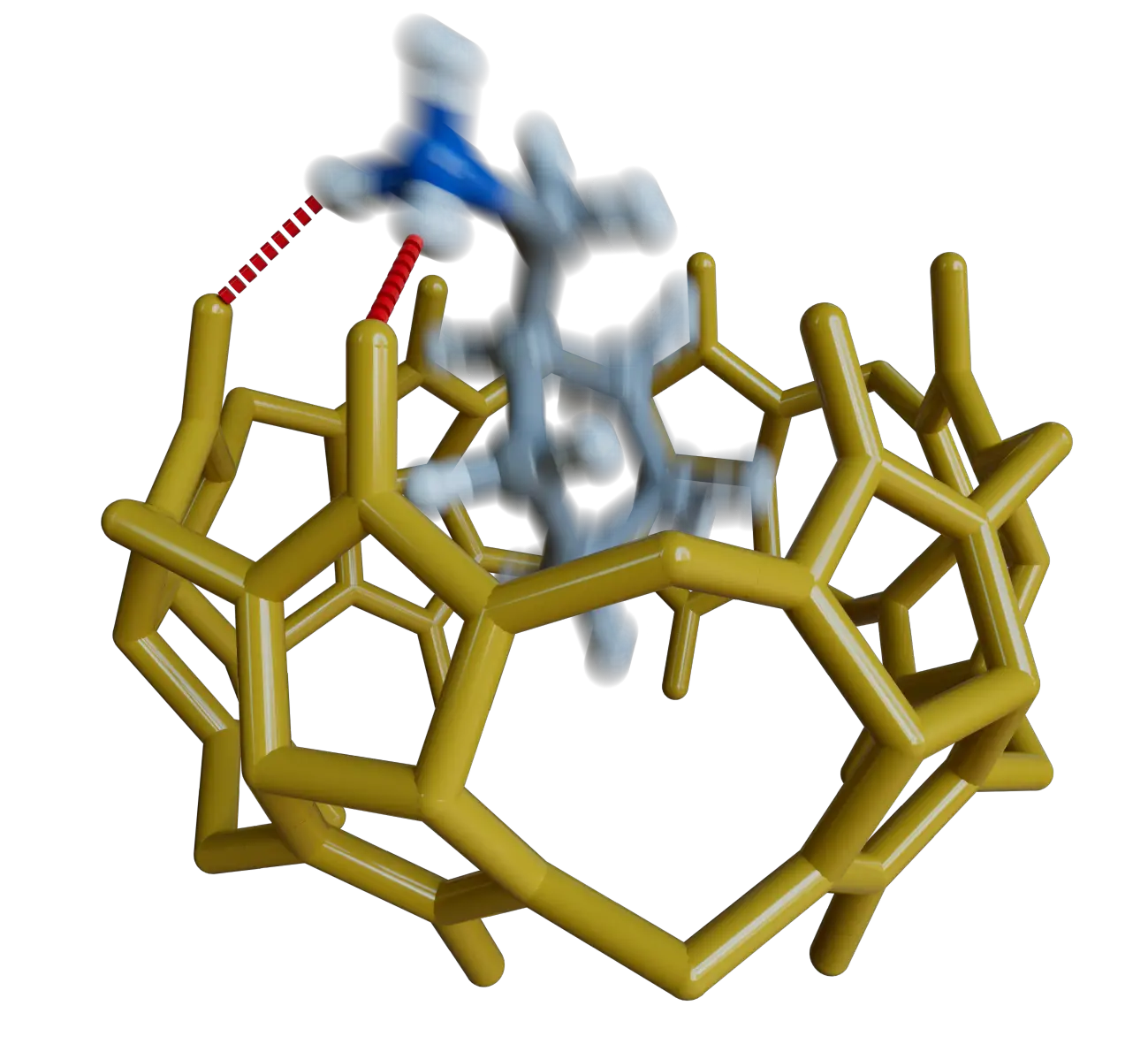
Gasevic, T.; Plett, C.; Wittmann, L.; Neira, I.; Peinador, C.; García, M. D.; Hansen, A. Supramolecular Host‐Guest Complexation Dynamics by Cost‐Efficient Electronic Structure Methods Chem. Eur. J. 2025, , accepted. DOI: 10.1002/chem.202502300
Multilevel computational approaches based on electronic structure methods currently enable the accurate structural and thermodynamic characterization of medium to large-sized host–guest systems, but have been scarcely used to systematically study their kinetics. Here, we show how this type of protocol can be easily applied for the task, using the well-known complexation reaction between the cucurbit[6]uril host and alkylammonium cations as a test case. To this end, the recently reported aISS docking workflow is used for the fast exploration of the coconformational space of a set of nine host–guest aggregates, at the robust and fast semiempirical GFN2-xTB(ALPB) level of theory. After identification of appropriate intermediates and reaction products, those can be in turn efficiently connected by the Nudged Elastic Band method, enabling fast screening of different potential reaction mechanisms, and the identification of the corresponding transition states. Further refinement of the equilibrium geometries and a better energetic description of the stationary points is nevertheless mandatory, for instance with the state of the art and efficient ωB97X-3c(COSMO-RS)//r2SCAN-3c(CPCM) density functional-based protocol, which not only accurately reproduces the experimental reference values of the free energy of association (MAE = 1.4 kcal/mol), but also the kinetic in/out barriers reported for the processes (MAE = 2.0 and 2.9 kcal/mol, respectively). Overall, this work presents a robust and cost-effective multilevel workflow for the routine kinetic profiling of supramolecular association processes.
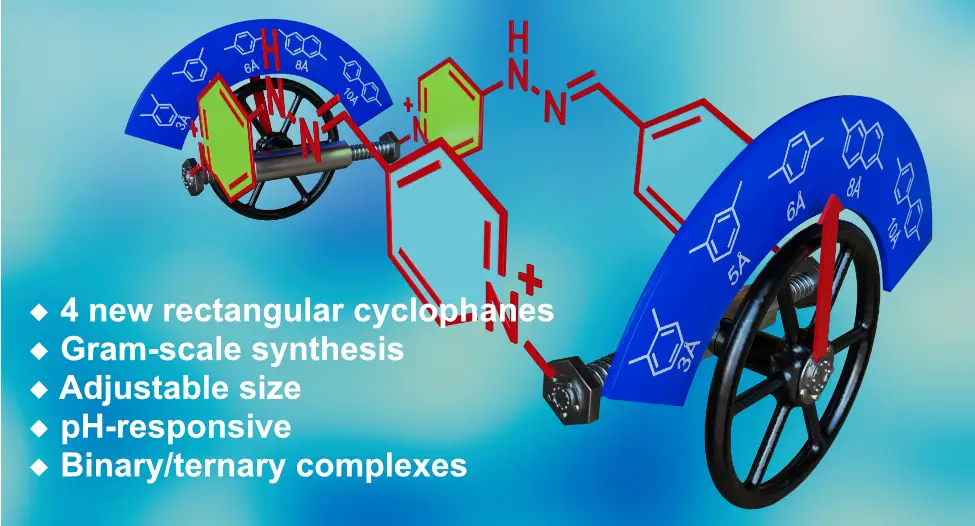
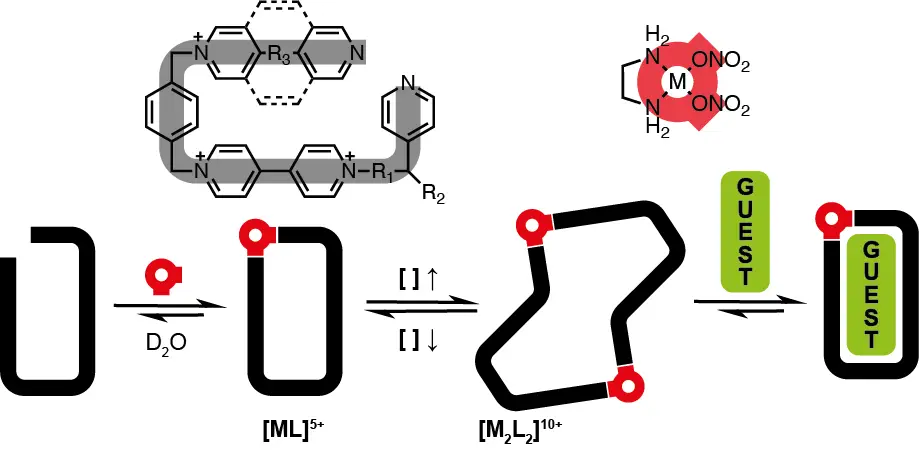
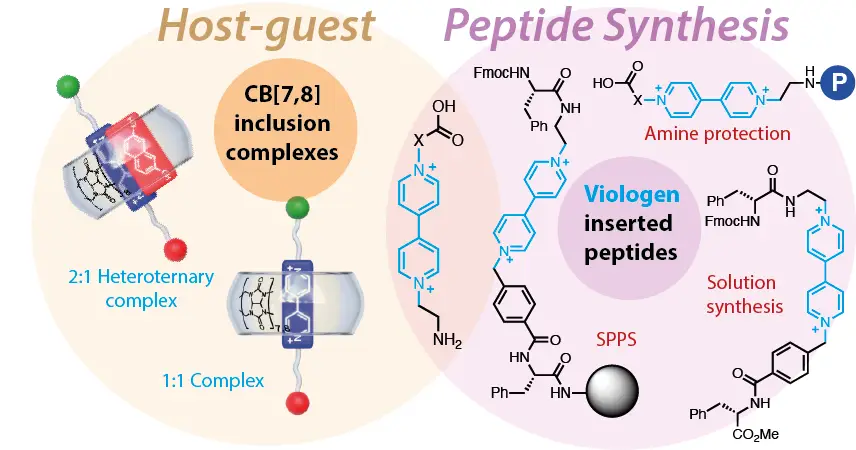
Fernández-Labandeira, N.; Montes de Oca, I.; Pazos, E.; Blanco-Gómez, A.; Peinador, C.; García, M. D. Modulating the Dimensions of Rectangular Hydrazone-Based Bispyridinium Macrocyclic Receptors. J. Org. Chem. 2025, 90, 8621-8627. DOI: 10.1021/acs.joc.5c00695
The development of new macrocyclic molecular receptors has driven major advances in supramolecular chemistry. However, realizing the full potential of host–guest systems requires addressing persistent challenges such as improved synthesis, aqueous performance, and implementation of stimuli-responsiveness. Herein, we present a new family of self-assembled polycationic molecular rectangles, derived from our previously reported redbox host. These novel cyclophanes share a common structural core with the parent compound─two pyridinium units linked by a hydrazone bond─but are designed with varying dimensions on the short sides, separating the bispyridinium walls. Synthesized via acid-catalyzed imine condensation reactions in water, these compounds were obtained in acceptable yields in gram scale through a modular and highly efficient approach. The aqueous molecular recognition properties of these new hosts were investigated using NMR spectroscopy with a 1,5-dihydroxynaphthalene derivative as a model aromatic electron donor. These studies demonstrate the formation of highly stable binary or ternary inclusion complexes in aqueous media, highlighting the tunability of the binding properties and applicative potential of this family of pH-responsive macrocyclic receptors.
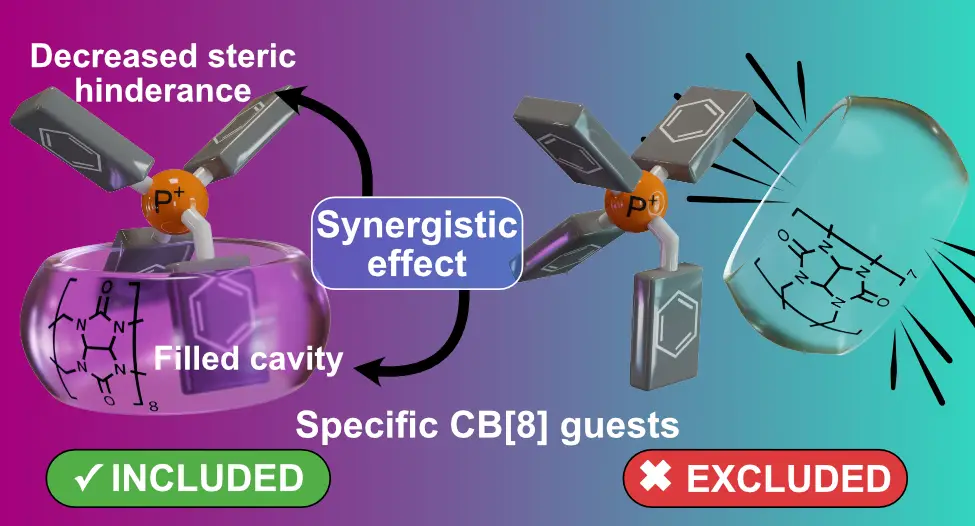
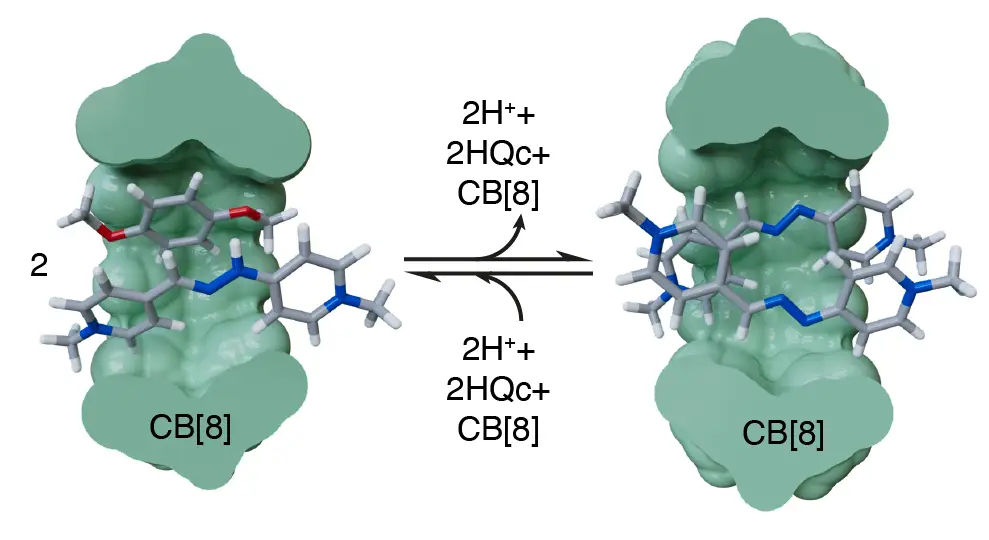
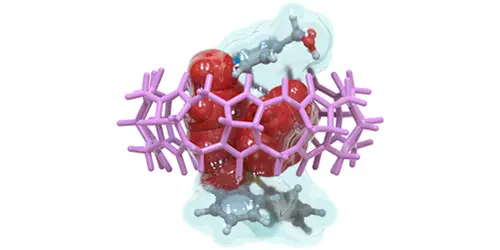
Díaz-Abellás, M.; Neira, I.; Blanco-Gómez, A.; Peinador, C.; García, M. D. Synergy-Promoted Specific Alkyltriphenylphosphonium Binding to CB[8] J. Org. Chem. 2025, 90, 4149-4157. DOI: 10.1021/acs.joc.4c02546
Biological substrate specificity ensures that organisms interact accurately with biomolecular receptors, crucial for key functions such as signaling and immunity. Nevertheless, this phenomenon is still poorly understood, with host–guest chemistry offering a suitable platform for studying simplified models. Herein, we report an in-depth study of the host–guest chemistry of alkyltriphenylphosphonium cations with cucurbit[8]uril (CB[8]), initiated by the serendipitous discovery of salt forming a tightly bound pseudoheteroternary 1:1 complex with CB[8]. A first generation of model substrates was designed to explore an unusual binding mode characterized by the simultaneous introduction of two distinct guest fragments within the host cavity. Structural features of the complexes were elucidated using ESI-MS and NMR 1D/2D techniques; thermodynamic properties were assessed by isothermal titration calorimetry, and kinetic parameters were derived from selective inversion–recovery NMR. Experimental results aligned well with electronic structure calculations, revealing a reproducible binding motif with submicromolar affinities. This peculiar complexation mode involves a synergistic effect caused by steric crowding around the P+ atom, facilitating the insertion of two aromatic units into CB[8] while hindering association with CB[7]. Based on these findings, a second generation of minimalistic substrates was developed, preserving the synergistic interaction mode and exhibiting specific binding to CB[8].