Reseach
Research in Supramolecular Engineering group is focussed in the development and application of new host-guest systems, molecular interlocked structures, and macrocyclic receptors based on non-covalent interactions and dynamic covalent chemistry.
Stoddart’s “blue box” (B), is one of the most iconic molecules in the recent history of chemistry. This rectangular tetracationic cyclophane has not only the ability to complex a wide variety of aromatic guests in organic or aqueous media but, due to the presence of viologen units on its structure, it also behaves as a redox-based molecular switch. In turn, B-based host-guest aggregates can translate this responsiveness from the molecular to the supramolecular level, resulting in host-controlled binding. This unique behaviour has allowed the development of a wide variety of B-containing (supra)molecular switches and machines, which certainly have inspired a whole generation of supramolecular chemists. Nevertheless, issues such as synthetic accessibility, structural diversity or the implementation of new chemical properties (luminescence, pH or photoresponsiveness, etc.), have restricted somehow the development of new practical applications in the ever-changing realm of modern host-guest chemistry.
Based largely on our own research throughout the last decade, we have developed two different strategies for the self-assembly of new B analogues: (1) Pd(II)/Pt(II) metal-directed self-assembly, (2) imine-based dynamic covalent chemistry. In essence, the strategies are based on the substitution of inert C-C single bonds on the macrocycle by Pd/Pt-N or C=N bonds of modifiable lability. In the case of the metal-directed synthesis, the use of Pd(II) centers allows for the spontaneous self-assembly at r.t., either in organic or aqueous media, of N-alkyl-4,4’-bipyridinium-based ligands into the desired metallacycles. Conversely, more inert Pt(II) salts can be also implemented, rendering the synthesis of more kinetically stable analogues.
Alternatively, wholly organic B congeners can be produced in a modular fashion by using imine-based dynamic covalent chemistry, allowing for the self-assembly in acidic water of macrocyclic pH-responsive molecular switches of adjustable kinetic stability.
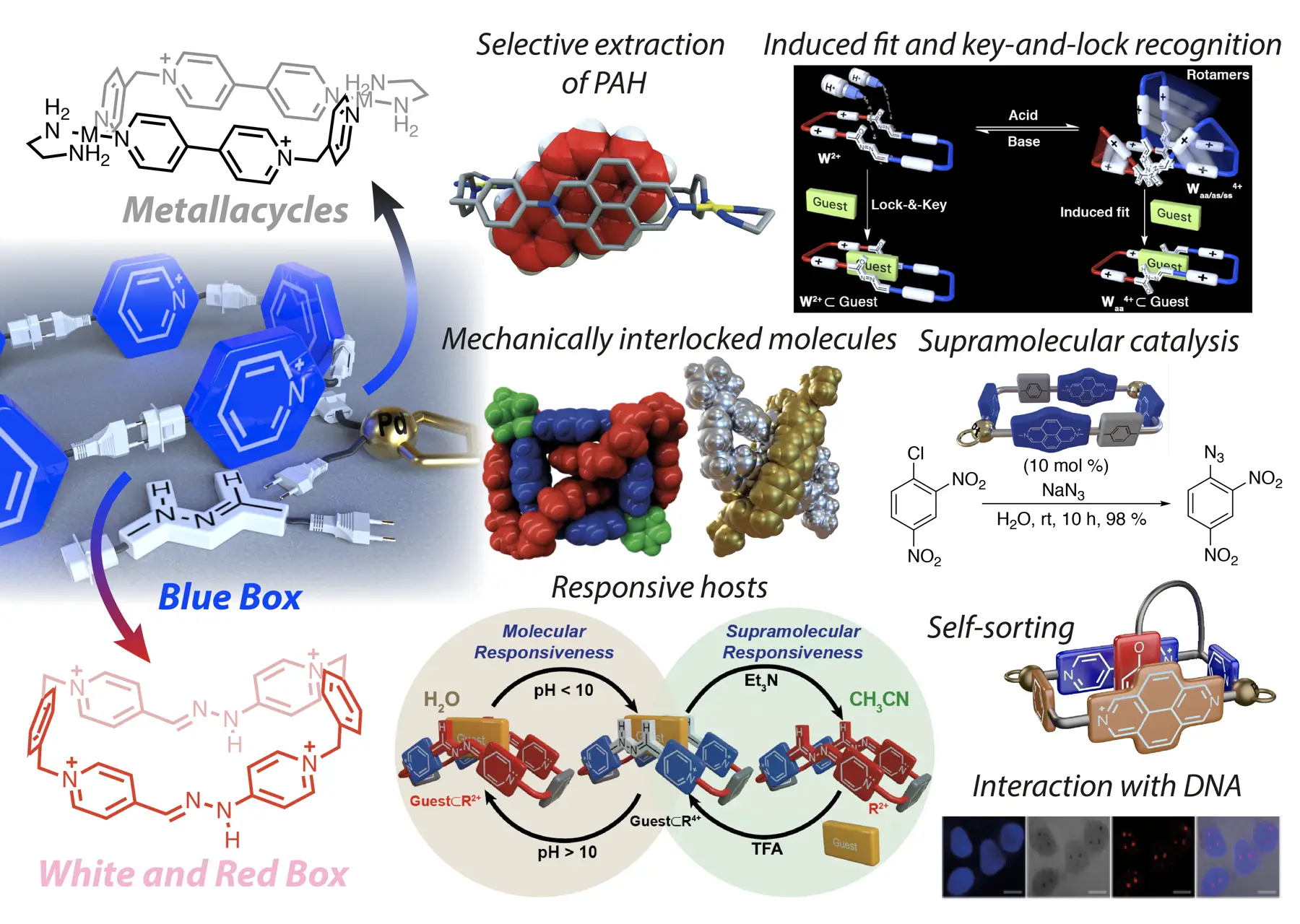
Owning pyridinium-based cavities of appropriate size, our B-inspired cyclophanes are able to complex aromatic substrates by a conjunction of the hydrophobic effect and π-π/C-H···π interactions. Consequently, these host-guest complexes can be achieved using our cyclophanes. Considering this knowledge, the implementation of our B-based macrocycles onto mechanically-interlocked molecules and knots was achieved, as well as the development of practical applications for the hosts in currently important research fields, such as the development of duplex and G4-DNA binders, supramolecular catalysis or the sequestration of relevant pollutants. Finally, self-assembled hosts offer the unique opportunity to include constitutional dynamism into host-guest chemistry, so examples of the development by our group of stimuli-responsive constitutionally dynamic libraries and self-sorted systems are also highlighted in the figure.