2020-2011 Publications
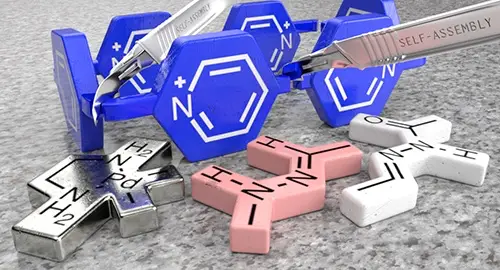
Neira, I.; Blanco-Gomez, A.; Quintela, J. M.; Garcia, M. D.; Peinador, C. Dissecting the "Blue Box": Self-Assembly Strategies for the Construction of Multipurpose Polycationic Cyclophanes Acc. Chem. Res. 2020, 53, 2336-2346. DOI: 10.1021/acs.accounts.0c00445
Stoddart's "blue box" (B(4+)), is one of the most iconic molecules in the recent history of chemistry. This rectangular tetracationic cyclophane has not only the ability to complex a wide variety of aromatic guests in organic or aqueous media, but because of the presence of viologen units on its structure, it also behaves as a redox-based molecular switch. In turn, B(4+)-based host-guest complexes can translate this responsiveness from the molecular to the supramolecular level, resulting in host-controlled binding. This unique behavior has allowed the development of a wide variety of B(4+)-containing (supra)molecular switches and machines, which certainly have inspired a whole generation of supramolecular chemists. Nevertheless, issues, such as synthetic accessibility, structural diversity, or the implementation of new chemical properties (luminescence, pH- or photo-responsiveness, etc.), have restricted somehow the development of new practical applications in the ever-changing realm of modern host-guest chemistry.Based largely on our own research throughout the past decade, we will highlight in this account two different strategies for the self-assembly of new B(4+) analogues: (1) Pd(II)/Pt(II) metal-directed self-assembly and (2) hydrazone-based dynamic covalent chemistry. In essence, the strategies are based on the substitution of inert C-C single bonds on the macrocycle by Pd/Pt-N or C horizontal lineN bonds of modifiable lability. In the case of the metal-directed synthesis, the use of Pd(II) centers allows for the spontaneous self-assembly at r.t., either in organic or aqueous media, of N-alkyl-4,4'-bipyridinium-based ligands into the desired metallacycles. Conversely, more inert Pt(II) salts can be also implemented, rendering the synthesis of more kinetically stable analogues. Alternatively, wholly organic B(4+) congeners can be produced in a modular fashion by using hydrazone-based dynamic covalent chemistry, allowing for the self-assembly in acidic water of macrocyclic pH-responsive molecular switches of adjustable kinetic stability.Owning pyridinium-based cavities of appropriate size, our B(4+)-inspired cyclophanes are able to complex aromatic substrates by a conjunction of the hydrophobic effect and pi-pi/C-H...pi interactions. Consequently, we will discuss in detail the different host-guest complexes that can be achieved using our cyclophanes. Considering this knowledge, the implementation of our B(4+)-based macrocycles onto mechanically interlocked molecules and knots will be introduced, as well as the development of practical applications for the hosts in currently important research fields, such as the development of duplex and G4-DNA binders, supramolecular catalysis or the sequestration of relevant pollutants. Finally, self-assembled hosts offer the unique opportunity to include constitutional dynamism into host-guest chemistry, so examples of the development by our group of stimuli-responsive constitutionally dynamic libraries and self-sorted systems will be highlighted.
Blanco-Gomez, A.; Corton, P.; Barravecchia, L.; Neira, I.; Pazos, E.; Peinador, C.; Garcia, M. D. Controlled binding of organic guests by stimuli-responsive macrocycles Chem. Soc. Rev. 2020, 49, 3834-3862. DOI: 10.1039/d0cs00109k
Synthetic supramolecular chemistry pursues not only the construction of new matter, but also control over its inherently dynamic behaviour. In this context, classic host-guest chemistry, based on the development of a myriad of macrocyclic receptors with fine-tuned affinities and selectivities, has enormously contributed to the discovery of new chemical function under self-assembly conditions. In turn, the use of molecular switches as control units within host-guest assemblies opened the door for the regulation of their dynamic interactional behaviour, which can be translated into controlled aggregation. In this review, we will focus on different strategies developed for the regulated binding of organic molecules by switchable macrocyclic hosts. As we will see, an appropriate design using stimuli-responsive versions of well-known organic receptors allows the molecular switches implemented within their structures to transform their regulated behaviour from the molecular to the supramolecular level.
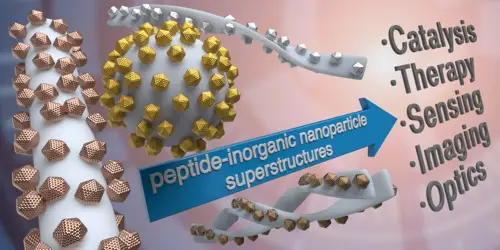
Pigliacelli, C.; Sanchez-Fernandez, R.; Garcia, M. D.; Peinador, C.; Pazos, E. Self-assembled peptide-inorganic nanoparticle superstructures: from component design to applications Chem. Commun. 2020, 56, 8000-8014. DOI: 10.1039/d0cc02914a
Peptides have become excellent platforms for the design of peptide-nanoparticle hybrid superstructures, owing to their self-assembly and binding/recognition capabilities. Morover, peptide sequences can be encoded and modified to finely tune the structure of the hybrid systems and pursue functionalities that hold promise in an array of high-end applications. This feature article summarizes the different methodologies that have been developed to obtain self-assembled peptide-inorganic nanoparticle hybrid architectures, and discusses how the proper encoding of the peptide sequences can be used for tailoring the architecture and/or functionality of the final systems. We also describe the applications of these hybrid superstructures in different fields, with a brief look at future possibilities towards the development of new functional hybrid materials.
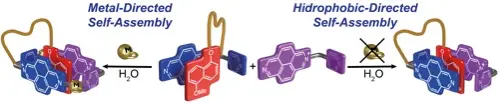
Rama, T.; Blanco-Gomez, A.; Peinador, C.; Garcia, M. D. Self-Assembly of Pseudo[1]rotaxanes by Palladium(II)/Platinum(II)-Directed Integrative Social Self-Sorting: Is the Metal Required? ChemPlusChem 2020, 85, 2672-2678. DOI: 10.1002/cplu.202000739
New results are presented on the multicomponent supramolecular synthesis of pseudo[1]rotaxanes, achieved by designing pairs of structurally matching N-monoalkyl-4,4'-bipyridinium/2,7-diazapyrenium-based ligands having complementary pi-donor/acceptor features, and intended to self-assemble into the targeted supramolecules by following integrative self-sorting processes. In all the studied cases, it was found that the envisioned species, characterized by NMR spectroscopy and MS spectrometry, arise as the main products of the self-assembly in aqueous media by using palladium(II)/platinum(II) metal centers as the guiding force. Crucially, we have also found that by improving the pi-donor/acceptor properties of the matching pairs of ligands (L4 and L5 ), the integrative self-sorting processes prevail even in the absence of metallic ions to afford the heterodimeric species with an association constant being 756+/-43 M(-1) .
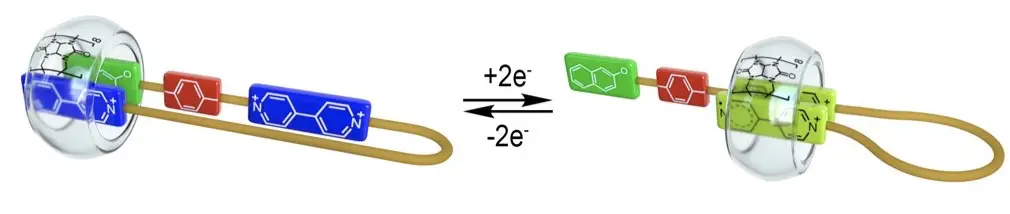
Neira, I.; Domarco, O.; Barriada, J. L.; Franchi, P.; Lucarini, M.; Garcia, M. D.; Peinador, C. An electrochemically controlled supramolecular zip tie based on host-guest chemistry of CB[8] Org. Biomol. Chem. 2020, 18, 5228-5233. DOI: 10.1039/d0ob01085e
Three molecular threads incorporating two viologen units attached by an oligo-ethylene glycol chain of variable length and an electron rich naphthalene moiety were prepared. The internal viologen unit is connected to the naphthalene by a rigid linker that prevents the simultaneous complexation of these systems. The axle with the shortest chain (14+) in the presence of CB[8] placed the chain inside the macrocycle in aqueous media. In contrast, the longer chains in 24+ and 34+ allow locating the terminal viologen and the electron rich unit inside the cavity. Upon reduction of the bipyridinium salts, the system behaves like a zip tie relaxing the chain as a consequence of the insertion of both radical cation moieties within the CB[8] and making these switches as potential components for molecular machinery.
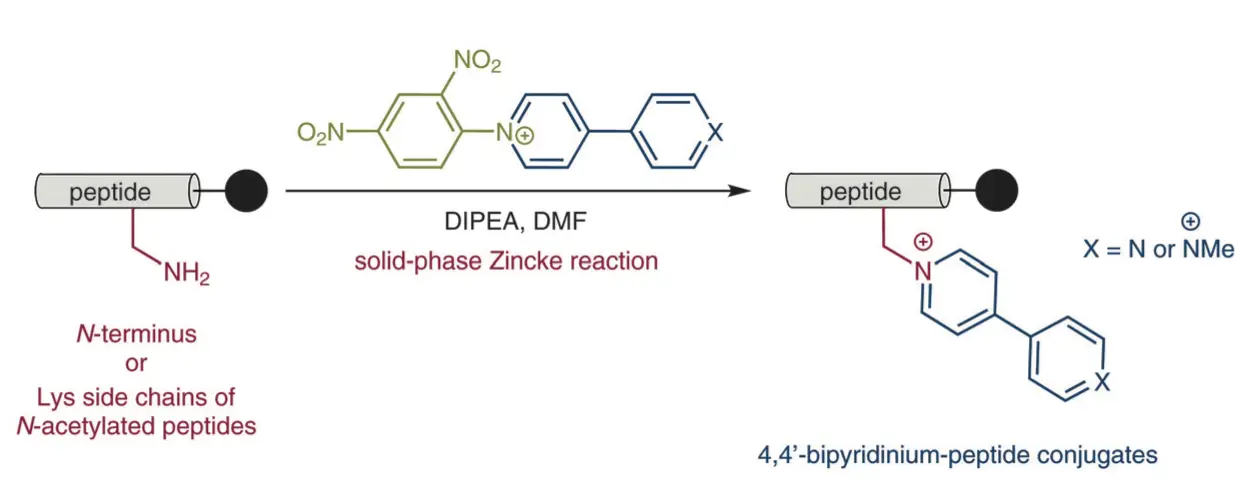
Corton, P.; Novo, P.; Lopez-Sobrado, V.; Garcia, M. D.; Peinador, C.; Pazos, E., Solid-Phase Zincke Reaction for the Synthesis of Peptide-4,4 '-bipyridinium Conjugates. Synthesis 2020, 52, 537-543. DOI: 10.1055/s-0039-1690016
We present herein the development of a new synthetic strategy for the conjugation of 4,4'-bipyridinium derivatives into peptide scaffolds. The methodology, based on the development of a solid-phase version of the Zincke reaction between activated pyridinium salts and amines, is able to produce the desired conjugates in a straightforward fashion, with the bipyridinium units attached at the N-terminus of peptides or at Lys side chains of N-terminal acetylated peptides.
Pazos, E.; Novo, P.; Peinador, C.; Kaifer, A. E.; Garcia, M. D., Cucurbit[8]uril (CB 8 )-Based Supramolecular Switches. Angew. Chem., Int. Ed. Engl. 2019, 58, 403-416. DOI: 10.1002/anie.201806575
The use of cucurbit[8]uril as a molecular host has emerged in the chemical literature as a reliable strategy for the creation of dynamic chemical systems, owing to its ability to form homo- and heteroternary complexes in aqueous media with appropriate molecular switches as guests. In this manner, CB[8]-based supramolecular switches can be designed in a predictable and modular fashion, through the selection of appropriate guests able to condition the redox, photochemical, or pH-triggered behavior of tailored multicomponent systems. Furthermore, CB[8] allows the implementation of dual/triple and linear/orthogonal stimuli-dependent properties into these molecular devices by a careful selection of the guests. This versatility in their design gives these supramolecular switches great potential for the rational development of new materials, in which their function is not only determined by the custom-made stimuli-responsiveness, but also by the transient aggregation/disaggregation of homo- or heteromeric building blocks.
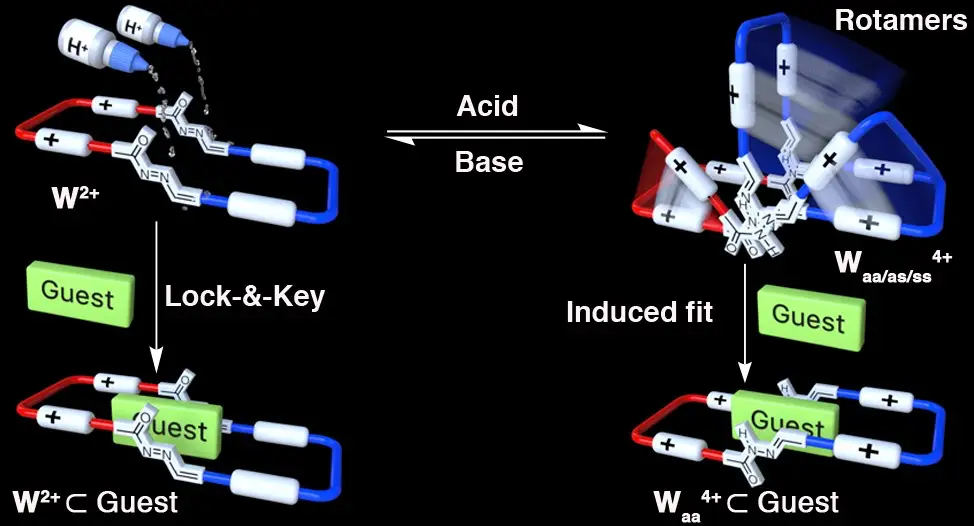
Blanco-Gomez, A.; Fernandez-Blanco, A.; Blanco, V.; Rodriguez, J.; Peinador, C.; Garcia, M. D., Thinking Outside the "Blue Box": Induced Fit within a Unique Self-Assembled Polycationic Cyclophane. J. Am. Chem. Soc. 2019, 141, 3959-3964. DOI: 10.1021/jacs.8b12599
We present herein the development of a new polycationic molecular receptor, inspired by the ubiquitous cyclobis(paraquat-p-phenylene)cyclophane ("blue box"). Our analogue, the "white box", has been easily self-assembled on a preparative scale in water, using a template-assisted process by acyl hydrazone bonding of complementary bis(pyridinium)xylylene tweezers, followed by kinetic trapping of the empty receptor. The obtained macrocycle was found to display a marked pH responsiveness in water, because of an abnormal acidity of the amide protons within its structure. Consequently, and because of the concurrence of rotational isomerism under acidic conditions (fixed at higher pH values), the compound was found to display a dual behavior as a conformationally locked/flexible molecular host, being able to recognize appropriate aromatic substrates, in a lock and key or induced fit fashion, by a conjunction of pi-pi, C-H center dot center dot center dot pi, and, crucially, the hydrophobic effect.
Domarco, O.; Kieler, C.; Pirker, C.; Dinhof, C.; Englinger, B.; Reisecker, J. M.; Timelthaler, G.; Garcia, M. D.; Peinador, C.; Keppler, B. K.; Berger, W.; Terenzi, A., Subcellular Duplex DNA and G-Quadruplex Interaction Profiling of a Hexagonal Pt-II Metallacycle. Angew. Chem., Int. Ed. Engl. 2019, 58, 8007-8012. DOI: 10.1002/anie.201900934
Metal-driven self-assembly afforded a multitude of fascinating supramolecular coordination complexes (SCCs) with applications as catalysts, host-guest, and stimuli-responsive systems. However, the interest in the biological applications of SCCs is only starting to emerge and thorough characterization of their behavior in biological milieus is still lacking. Herein, we report on the synthesis and detailed in-cell tracking of a Pt2L2 metallacycle. We show that our hexagonal supramolecule accumulates in cancer cell nuclei, exerting a distinctive blue fluorescence staining of chromatin resistant to UV photobleaching selectively in nucleolar G4-rich regions. SCC co-localizes with epitopes of the quadruplex-specific antibody BG4 and replaces other well-known G4 stabilizers. Moreover, the photophysical changes accompanying the metallacycle binding to G4s in solution (fluorescence quenching, absorption enhancement) also take place intracellularly, allowing its subcellular interaction tracking.
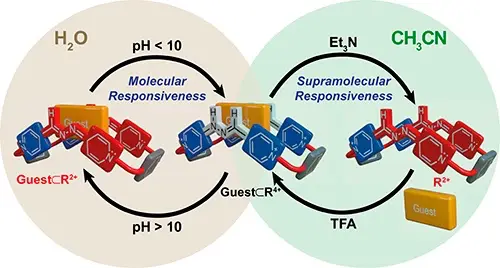
Blanco-Gomez, A.; Neira, I.; Barriada, J. L.; Melle-Franco, M.; Peinador, C.; Garcia, M. D., Thinking outside the "Blue Box": from molecular to supramolecular pH-responsiveness. Chem. Sci. 2019, 10, 10680-10686. DOI: 10.1039/c9sc04489b
We present herein the development of a new polycationic cyclophane: the "red box", second in a series of hydrazone-based analogues of the well-known organic receptor cyclobis(paraquat-p-phenylene)cyclophane ("blue box"). The macrocycle has been prepared in an excellent yield in aqueous media, and shows both a remarkable pH-responsiveness and unusual hydrolytic stability of the two hydrazone C[double bond, length as m-dash]N bonds, associated with charge delocalization of the amine lone pair. Whilst in aqueous media the "red box" is able to complex a variety of aromatic substrates, both in its acidic and basic form, in organic media the cyclophane is only able to capture those in the acidic form, resulting in supramolecular pH-responsiveness.

Neira, I.; Blanco-Gomez, A.; Quintela, J. M.; Peinador, C.; Garcia, M. D., Adjusting the Dynamism of Covalent Imine Chemistry in the Aqueous Synthesis of Cucurbit[7]uril-based [2]Rotaxanes. Org. Lett. 2019, 21, 8976-8980. DOI: 10.1021/acs.orglett.9b03377
The self-assembly of [2]rotaxane has been achieved in aqueous media at pD 4 by the simultaneous threading into CB[7] of an appropriate axle ended with acyl hydrazine groups and its concurrent capping with two molecules of a triphenylphosphonium aldehyde as stoppers. The dynamism of the rotaxane has been demonstrated under acidic conditions and can be diminished by solvent swapping, pH modulation, or, surprisingly, the removal of the carbonyl groups on the axle.

Neira, I.; Alvarino, C.; Domarco, O.; Blanco, V.; Peinador, C.; Garcia, M. D.; Quintela, J. M., Tuning of the Self-Threading of Ring-in-Ring Structures in Aqueous Media. Chem. Eur. J. 2019, 25, 14834-14842. DOI: 10.1002/chem.201902851
A series of aryl-extended N-monoalkyl-4,4'-bipyridinium salts L (aryl=1,4-phenyl, 4,4'-biphenyl, 2,6-naphthyl and 9,10-anthracenyl) have been implemented by Pd(II) /Pt(II) -directed self-assembly into constitutionally dynamic systems (CDSs). As a result, the intended processes produced not only (en)M2 L2 (en=ethylenediamine) metallacyclic species but also (en)M4 L4 ring-in-ring aggregates, in equilibrium with the former, as a consequence of the hydrophobic nature of the aryl rings within the 4,4'-bipyridinium scaffold. The key feature of the obtained dynamic systems is the possibility of modulating their response against external stimuli by modifying the hydrophobic character of the ligand. While the different dynamic libraries follow the same trends upon changes in concentration, temperature, polarity of the medium, or addition of an aromatic chemical effector, subtle changes in the ligand hydrophobic core results in a fine-tuning of the speciation when applying a certain degree of the different stimulus. The exception is the anthracene-containing derivative, which does not form inclusion complexes or self-threaded structures.
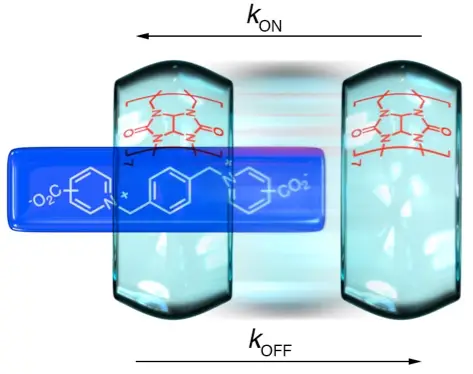
Neira, I.; Garcia, M. D.; Peinador, C.; Kaifer, A. E., Terminal Carboxylate Effects on the Thermodynamics and Kinetics of Cucurbit 7 uril Binding to Guests Containing a Central Bis(Pyridinium)-Xylylene Site. J. Org. Chem. 2019, 84, 2325-2329. DOI: 10.1021/acs.joc.8b02993
A series of bis(pyridinium)-xylylene derivatives bearing carboxylate terminal groups were investigated as guests for the cucurbit[7]uril host in aqueous solution. While the presence of the terminal carboxylates has a modest effect on the thermodynamic stability of the complexes, the kinetics of complex association/dissociation is strongly affected. The relative position (meta, para) of the carboxylate group in relation to the pyridinium nitrogen also exerts a considerable effect on the binding kinetics.
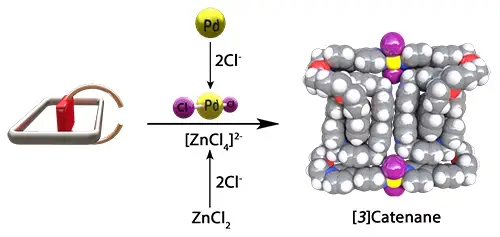
Lopez-Vidal, E. M.; Prokofjevs, A.; Gibbs-Hall, I. C.; Dale, E. J.; Quintela, J. M.; Peinador, C., Studies towards the synthesis of Pd(II)-containing 2 and 3 catenanes in aqueous media. Dalton Trans. 2018, 47, 2492-2496. DOI: 10.1039/c7dt04792d
Here is reported the investigation of a synthetic route for the preparation of Pd(II)-containing catenanes in aqueous media. A pseudorotaxane intermediate was prepared, which can potentially be converted into a series of catenanes. From the pseudorotaxane, using a Pd(II)-driven clipping step a dinuclear [3]catenane was obtained in the solid state.
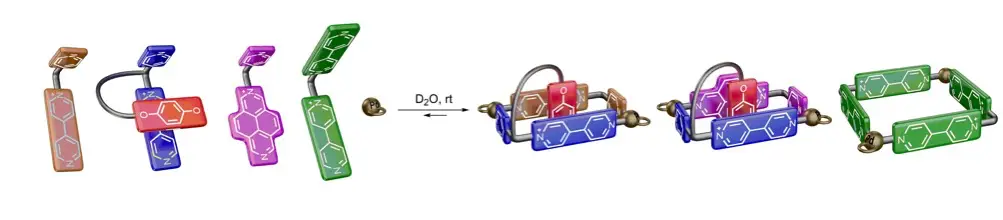
Rama, T.; Blanco-Gomez, A.; Neira, I.; Domarco, O.; Garcia, M. D.; Quintela, J. M.; Peinador, C., Integrative Self-Sorting of Bipyridinium/Diazapyrenium-Based Ligands into Pseudo[1]rotaxanes. Chem. Eur. J. 2017, 23, 16743-16747. DOI: 10.1002/chem.201704540
We present here the design and synthesis of a series of multicomponent supramolecular architectures, structures formed by the PdII/PtII-directed integrative social self-sorting in aqueous media of pairs of complementary N-monoalkyl-4,4′-bipyridinium/2,7-diazapyrenium-based ligands. Out of the different potential outcomes of the processes, we have found out how the designed systems selectively enhance the production of pseudo[1]rotaxanes, hermaphroditic host–guest aggregates that maximize the strength of the occurring π–π, C−H⋅⋅⋅π, and hydrophobic interactions, as well as the number of those interactions per receptor. It is also demonstrated how both integrative social and narcissistic PdII-directed self-sorting can occur orthogonally and concomitantly for this type of ditopic ligands.
Domarco, O.; Neira, I.; Rama, T.; Blanco-Gomez, A.; Garcia, M. D.; Peinador, C.; Quintela, J. M., Synthesis of non-symmetric viologen-containing ditopic ligands and their Pd(II)/Pt(II)-directed self-assembly. Org. Biomol. Chem. 2017, 15, 3594-3602. DOI: 10.1039/c7ob00161d
We present here an efficient method for the preparation of non-symmetric viologen-containing ditopic ligands, as well as the Pd(ii)/Pt(ii)-directed self-assembly of some of these into metallacyclic receptors. The designed synthetic route, that implies the sequential alkylation and the Zincke reaction of activated bipyridinium salts, allowed us to substantially improve the yield in the preparation of three previously reported ligands. The versatility and efficiency of the method have also been tested for the preparation of four new viologen-containing ligands with very different structural features. Furthermore, the self-assembly of the new ligands around Pd(ii) and Pt(ii) centers has been tested, yielding mononuclear rectangular-shaped metallacycles of different dimensions and electronic characteristics.

Marcos, I.; Domarco, O.; Peinador, C.; Fenandez, A.; Fernandez, J. J.; Vazquez-Garcia, D.; Garcia, M. D., Self-assembly of dinuclear Pd(II)/Pt(II) metallacyclic receptors incorporating N-heterocyclic carbene complexes as corners. Dalton Trans. 2017, 46, 4182-4190. DOI: 10.1039/c6dt04476j
We report herein the self-assembly of a series of new square and rectangular-shaped dinuclear M2L2 metallacycles (M = Pd(ii)/Pt(ii)), receptors self-assembled in water from four different N-monoalkyl-4,4'-bipyridinium derivatives as ligands and square-planar Pd(ii) and Pt(ii) metal centers having the chelating N-heterocyclic carbene 1,1'-di(methyl)-3,3'-methylene-4-diimidazolin-2,2'-diylidene. The concentration-dependent Pd2L2 metallacycles were successfully obtained and characterized by means of NMR experiments in aqueous media. Due to the strong trans effect exerted by the carbene ligands, the synthesis of the Pt2L2 receptors was achieved as well by self-assembly of the components at room temperature in a few hours, in clear contraposition to the harsh reaction conditions usually required for the labilization of other kinetically inert Pt(ii)-N(pyridine) bonds. X-ray diffraction studies of suitable single crystals of two of the obtained receptors offered additional information on the structure of the obtained supramolecules, whose ability as receptors has been explored by the preparation and study of the corresponding inclusion complexes in water with 1,5-dihydroxynaphthalene as the model substrate.
Domarco, O.; Lotsch, D.; Schreiber, J.; Dinhof, C.; Van Schoonhoven, S.; Garcia, M. D.; Peinador, C.; Keppler, B. K.; Berger, W.; Terenzi, A., Self-assembled Pt2L2 boxes strongly bind G-quadruplex DNA and influence gene expression in cancer cells. Dalton Trans. 2017, 46, 329-332. DOI: 10.1039/c6dt03876j
Supramolecular Pt(ii) quadrangular boxes bind native and G-quadruplex DNA motifs in a size-dependent fashion. Three Pt molecular squares of distinct size show biological activity against cancer cells and heavily influence the expression of genes known to form G-quadruplexes in their promoter regions. The smallest Pt-box displays less activity but more selectivity for a quadruplex formed in the c-Kit gene.
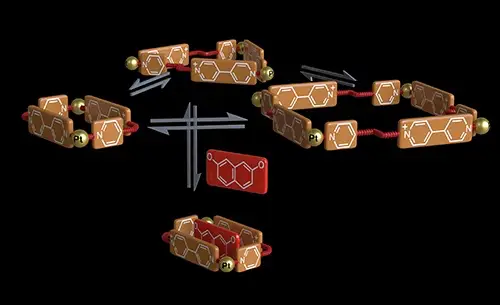
Blanco-Gomez, A.; Rama, T.; Domarco, O.; Neira, I.; Blanco, V.; Quintela, J. M.; Garcia, M. D.; Peinador, C., Amplification of a metallacyclic receptor out of a dynamic combinatorial library. Dalton Trans. 2017, 46, 15671-15675. DOI: 10.1039/c7dt03379f
We present herein the Pt(II)-directed self-assembly in water of a new conformationally flexible N-monoalkyl-4,4-bipyridinium-based ditopic ligand into a library of six different metallacyclic structures. This constitutionally dynamic library can be pushed to increase the production of one of the supramolecules in aqueous media, either by controlling the concentration of the building blocks or upon addition of an appropriate aromatic substrate.
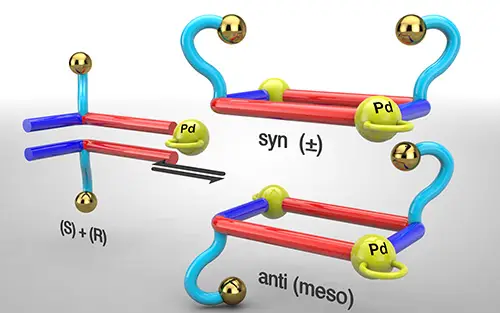
Rama, T.; Alvarino, C.; Domarco, O.; Platas-Iglesias, C.; Blanco, V.; Garcia, M. D.; Peinador, C.; Quintela, J. M., Self-assembly of Pd2L2 Metallacycles Owning Diversely Functionalized Racemic Ligands. Inorg. Chem. 2016, 55, 2290-2298. DOI: 10.1021/acs.inorgchem.5b02650
We present herein the efficient palladium(II)-directed self-assembly in water of a series of nine new diversely functionalized metallacycles, owning hydroxy/alkoxycarbonyl/azidoalkyl exo pendant groups attached to ditopic N-monoalkyl/aryl-4,4'-bipyridinium/2,7-diazapyrenium ligands. The highly convergent and versatile synthetic route for the ligands uses the Zincke reaction between (dinitrophenyl)-bipyridinium/diazapyrenium salts and racemic amines as the key step. The stereochemical outcome of the self-assembly of the Pd2L2 species is discussed on the basis of density functional theory quantum-chemical calculations.
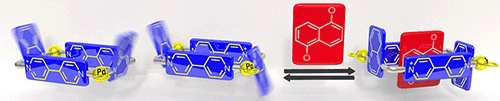
Blanco, V.; Abella, D.; Rama, T.; Alvarino, C.; Garcia, M. D.; Peinador, C.; Quintela, J. M., Guest-induced stereoselective self-assembly of quinoline-containing Pd-II and Pt-II metallacycles. Rsc Advances 2016, 6, 80181-80192. DOI: 10.1039/c6ra14909j
A family of mono- and dinuclear metallacycles has been self-assembled from (en)M(NO3)2 metal centers (M = PdII or PtII) and 4,4′-bipyridinium/2,7-diazapyrenium-based ligands incorporating quinoline subunits in their structures. These receptors recognize aromatic substrates, up to the dimensions of naphthalene, by means of hydrophobic forces, π–π stacking and C–H⋯π interactions. In the case of the M2L2 receptors, the quinoline moieties in the short sides of these molecular rectangles, cause the existence of two atropisomers with different cavity characteristics. The formation of inclusion complexes, derived from the dinuclear hosts and appropriate aromatics, induced stereoselectivity on the self-assembly, which was found to be modulated mainly by the size of the guests and their ability to optimize the number of potential C–H⋯π interactions with the two isomeric receptors. In addition, the π-acceptor and hydrophobic character of the host also contributes to this stereoselectivity. The proposed mechanism for the interconversion of the syn/anti isomers, studied by DFT methods, involves the rotation of the quinoline rings without dissociation of the self-assembled metallacycles.
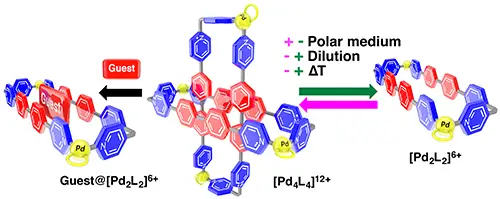
Alvarino, C.; Platas-Iglesias, C.; Blanco, V.; Garcia, M. D.; Peinador, C.; Quintela, J. M., Stimuli-responsive metal-directed self-assembly of a ring-in-ring complex. Dalton Trans. 2016, 45, 11611-11615. DOI: 10.1039/c6dt01835a
Concentration, temperature and/or solvent polarity control the speciation on the metal-directed self-assembly of a ditopic pyridyl ligand L with cis-protected Pd(II) metal centers. This results into a controllable dynamic system, involving a [Pd2L2](6+) metallacycle and a [Pd4L4](12+) ring-in-ring complex.
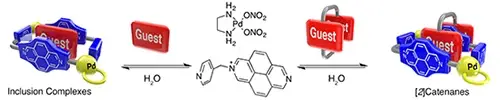
Rama, T.; Lopez-Vidal, E. M.; Garcia, M. D.; Peinador, C.; Quintela, J. M., Complexation and Catenation in Aqueous Media Using a Self-Assembled PdII Metallacyclic Receptor. Chem. Eur. J. 2015, 21, 9482-9487. DOI: 10.1002/chem.201501087
A M2L2 rectangular-shaped metallacycle, obtained by metal-directed self-assembly of a 2-(pyridin-4-ylmethyl)-2,7-diazapyrenium salt and [(en)Pd (NO3)(2)] (en=ethylenediamine), has been investigated as a molecular receptor for a wide range of aromatic substrates in water. Complexation and catenation of the receptor with selected mono- and polycyclic aromatic substrates produced 1:1 inclusion complexes and [2]catenanes in a highly efficient fashion, as determined by NMR and UV/Vis spectroscopic techniques, as well as single-crystal X-ray crystallography. Furthermore, the thermodynamic and kinetic features of the complexation processes have been analyzed for selected model guests.

Lopez-Vidal, E. M.; Garcia, M. D.; Peinador, C.; Quintela, J. M., When Self-Assembly Fails: Stepwise Metal-Directed Synthesis of [2]Catenanes. Chem. Eur. J. 2015, 21, 2259-2267. DOI: 10.1002/chem.201405297
On the attempted synthesis of a series of homo- and heterotrimetallic [2] catenanes by the self-assembly of a 2-(pyridin-4-ylmethyl)-2,7-diazapyrenium ligand, (ethylenediamine) palladium(II) or platinum(II) nitrate, and a dioxoaryl bis(N-monoalkyl-4,4'-bipyridinium) salt as building blocks, both the one-pot direct self-assembly of the components and the so called "magic ring" approach fail to produce the expected trinuclear [2] catenanes under thermodynamically driven conditions. However, one of the target supramolecules is obtained by following a stepwise protocol, consisting of the threading of a dinuclear Pt-II metallacycle and the dioxoaryl bis(N-monoalkyl-4,4'-bipyridinium) axle, followed by kinetically controlled Pt-II-directed cyclization of the corresponding pseudorotaxane.
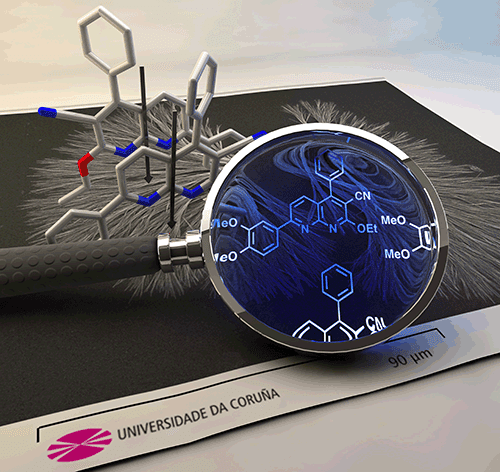
Fernandez-Mato, A.; Sanchez-Andujar, M.; Pato-Doldan, B.; Senaris-Rodriguez, M. A.; Platas-Iglesias, C.; Tordera, D.; Bolink, H. J.; Quintela, J. M.; Peinador, C.; Garcia, M. D., Spontaneous Self-Assembly of a 1,8-Naphthyridine into Diverse Crystalline 1D Nanostructures: Implications on the Stimuli-Responsive Luminescent Behaviour. Cryst. Growth Des. 2014, 14, 3849-3856. DOI: 10.1002/chem.201405297
The previously reported organic solid-state fluorophore 7-(3,4-dimethoxyphenyl)-2-ethoxy-4-phenyl-1,8-naphthyridine-3-carbonitrile 1 was found to spontaneously self-organize into diverse 1D crystalline nanostructures by choosing appropriate liquid phase self-assembly conditions. Experimental results, as well as DFT quantum calculations (at the M06-2X/6-31+G(d) level), shed light on the aggregation mechanism. This was found in good agreement with molecules being primarily joined together through intermolecular alignment caused by electrostatic interactions, as well as minimization of the steric repulsions. This alignment provokes the preferential growth of the crystalline materials into 1D aggregates, as well as the observed fluorescence of the material in the solid state. Luminescent nanowires, nanodendrons, and nanodendrimers, obtained following this spontaneous liquid phase self-assembly, have shown a versatile fluorescent behavior. Here, the obtained nanostructured materials showed a blue-shifted emission compared to the previously reported polycrystalline or single crystalline samples of 1 (Pbca), together with thermo- and acido-fluorochromism, plus piezochromic aggregation-induced emission.
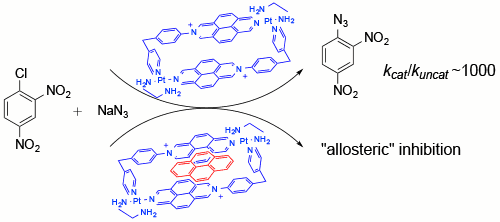
Lopez-Vidal, E. M.; Fernandez-Mato, A.; Garcia, M. D.; Perez-Lorenzo, M.; Peinador, C.; Quintela, J. M., Metallacycle-Catalyzed SNAr Reaction in Water: Supramolecular Inhibition by Means of Host-Guest Complexation. J. Org. Chem. 2014, 79, 1265-1270. DOI: 10.1021/jo402689p
The performance of a Pt-II diazapyrenium-based metallacycle as a reusable substoichiometric catalyst for the SNAr reaction between halodinitrobenzenes and sodium azide at it in aqueous media is reported. The results suggest that the catalytic effect is promoted by the association of the azide to the diazapyrenium cationic subunits of the catalyst. The findings demonstrate that the formation of an inclusion complex between pyrene and the metallacycle has a regulatory effect over the system, resulting in allosteric-like inhibition of the SNAr reaction.
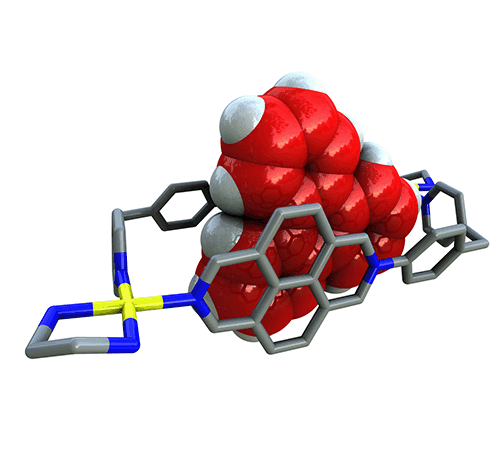
Garcia, M. D.; Alvarino, C.; Lopez-Vidal, E. M.; Rama, T.; Peinador, C.; Quintela, J. M., Complexation of aromatic compounds with self-assembled PdII and PtII metallacycles. Inorg. Chim. Acta 2014, 417, 27-37. DOI: 10.1016/j.ica.2013.10.037
Due to their stereoelectronic characteristics and flexible design, 2D self-assembled metallacyclic supramolecules can be considered as optimum potential receptors for aromatic systems. In the present account we focus on our contributions to the field of host-guest chemistry of metallacyclic Pd-II/Pt-II-receptors. It will be shown how effective complexation of aromatic substrates, including some of environmental significance, can be achieved by a careful selection of the ligands and metal centers involved in the self-assembly processes
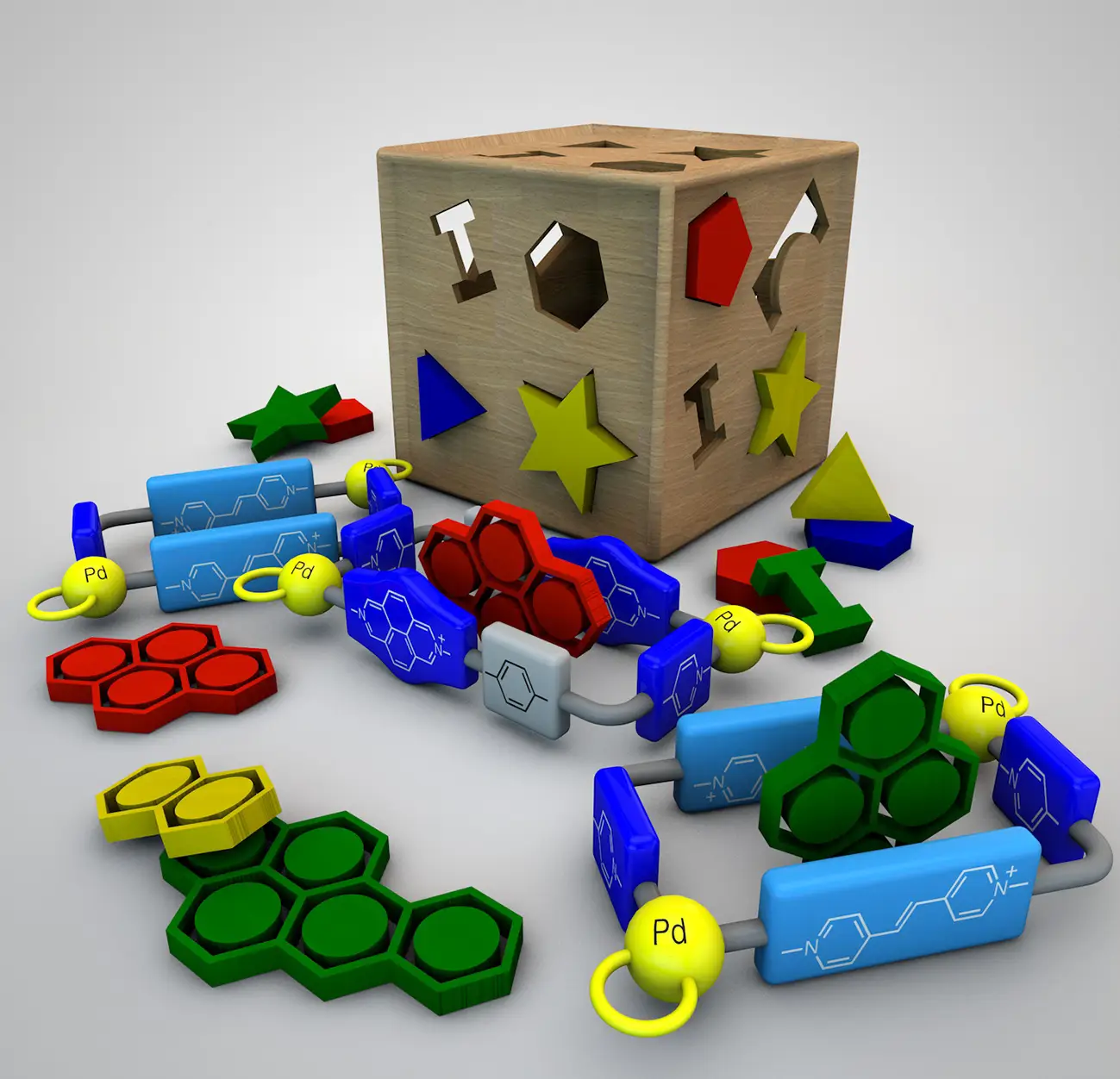
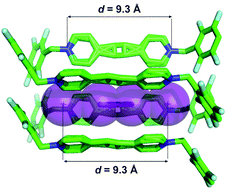
Alvariño, C.; Pía, E.; García, M. D.; Blanco, V.; Fernández, A.; Peinador, C.; Quintela, J. M., Dimensional matching of polycyclic aromatics with rectangular metallacycles: Insertion modes determined by [C-H···π] interactions. Chem. Eur. J. 2013, 19, 15329-15335. DOI: 10.1002/chem.201302165
A family of PdII/PtII dinuclear receptors, designed to give a smooth increase in their cavity lengths (from 7.46–13.78 Å), is presented. Their inclusion complexes with a representative set of polycyclic aromatic substrates (naphthalene, carbazol, pyrene, and benzo[a]pyrene), were characterized and studied in aqueous solution and the solid state. By taking into account the dimensions of both receptors and substrates, an excellent complementarity was found between the size of the receptors and their ability to complex a given substrate. Furthermore, this dimensional matching results in specific binding modes depending on the ability of the guest to establish stabilizing [C-H⋅⋅⋅π] interactions with the host.
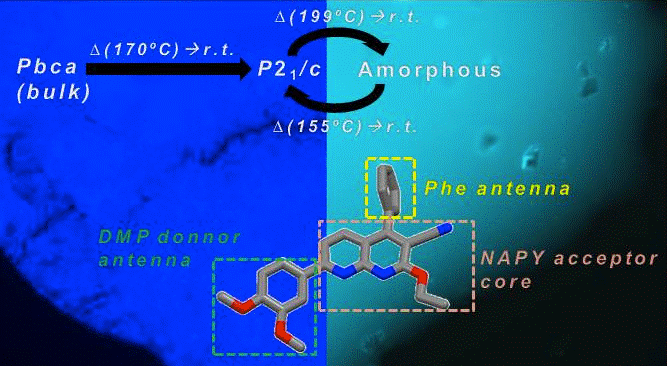
Fernandez-Mato, A.; Garcia, M. D.; Peinador, C.; Quintela, J. M.; Sanchez-Andujar, M.; Pato-Doldan, B.; Senaris-Rodriguez, M. A.; Tordera, D.; Bolink, H. J., Polymorphism-Triggered Reversible Thermochromic Fluorescence of a Simple 1,8-Naphthyridine. Cryst. Growth Des. 2013, 13, 460-464. DOI: 10.1021/cg301656x
The fluorescent behavior in the solid state of a naphthyridine-based donor-acceptor heterocycle is presented. Synthesized as a cryst. blue-emissive solid (Pbca), the compd. can easily be transformed in its P21/c polymorphic form by heating. The latter material shows blue to cyan emission switching triggered by a reversible thermally induced phase transformation. This fact, the reversible acidochromism, and the strong anisotropic fluorescence of the compd. in the solid state, account for the potential of 1,8-naphthyridines as simple and highly tunable org. compds. in materials science.
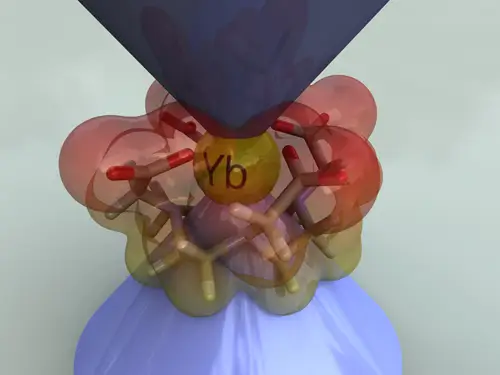
Lopez-Vidal, E. M.; Regueiro-Figueroa, M.; Garcia, M. D.; Platas-Iglesias, C.; Peinador, C.; Quintela, J. M., Probing Electrostatic Potential by NMR with the Use of a Paramagnetic Lanthanide(III) Chelate. Inorg. Chem. 2012, 51, 4429-4431. DOI: 10.1021/ic300288j
The paramagnetic complex [Yb(DOTA)](-) forms ion pairs in aqueous solution with cationic species such as N-monoalkyl- and N,N'-dialkyl-4,4'-bipyridinium cations. The magnitude and sign of the induced H-1 NMR pseudocontact shift values can be correlated to the electrostatic potential calculated at the MPWLYP/6-311G** level.
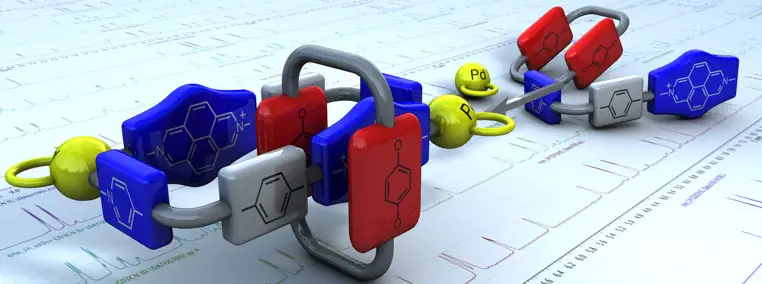
Alvarino, C.; Terenzi, A.; Blanco, V.; Garcia, M. D.; Peinador, C.; Quintela, J. M., [2]Catenanes and inclusion complexes derived from self-assembled rectangular PdII and PtII metallocycles. Dalton Trans. 2012, 41, 11992-11998. DOI: 10.1039/c2dt31116j
New inclusion complexes and [2]catenanes were self-assembled from a fluorescent diazapyrenium based ligand, a PdII or PtII complex, and cyclic or acyclic electron rich aromatic guests in aqueous and organic media. The molecular rectangles display a π-deficient cavity suitable to incorporate π-donor aromatic systems. The inclusion complexes between the metallocycles and phenylenic (2a,b) and naphthalenic (3a,b–5a,b) derivatives were studied by NMR, UV-vis and fluorescence spectroscopy. The crystal structure of (3b)⊂1a·6PF6 confirmed the insertion of the guest into the cavity of the metallocycle. Following the same self-assembly strategy, the use of polyethers 6,7 as π-donors resulted in the self-assembly of the [2]catenanes 1a(6,7)·6PF6. Single-crystal X-ray analysis of 1a(7)·6PF6 revealed the [2]catenane structure being stabilized by π-stacking and [C–H⋯O] interactions.
Lopez-Vidal, E. M.; Blanco, V.; Garcia, M. D.; Peinador, C.; Quintela, J. M., Synthesis of Platinum(II) Metallocycles Using Microwave-Assisted Heating. Org. Lett. 2012, 14, 580-583. DOI: 10.1021/ol203191b
An efficient microwave-assisted self-assembly of dinuclear platinum metallocycles is reported. The reactions proceed to afford the products with high purity and yields within 3-4 h. We have used this methodology to synthesize a new 14.75 angstrom x 14.75 angstrom molecular square. The solid-state structure showed a perfect alignment of the squares along the c axis.
Peinador, C.; Blanco, V.; García, M. D.; Quintela, J. M., PdII and PtII Metal-Directed Self-Assembly of Supramolecular Structures Based on N-Monoalkyl-4,4’-bipyridinium Derivatives In Molecular Self-Assembly - Advances and Applications, Li, A., Ed. Pan Stanford Publishing: Singapore, 2012; pp 347-378.
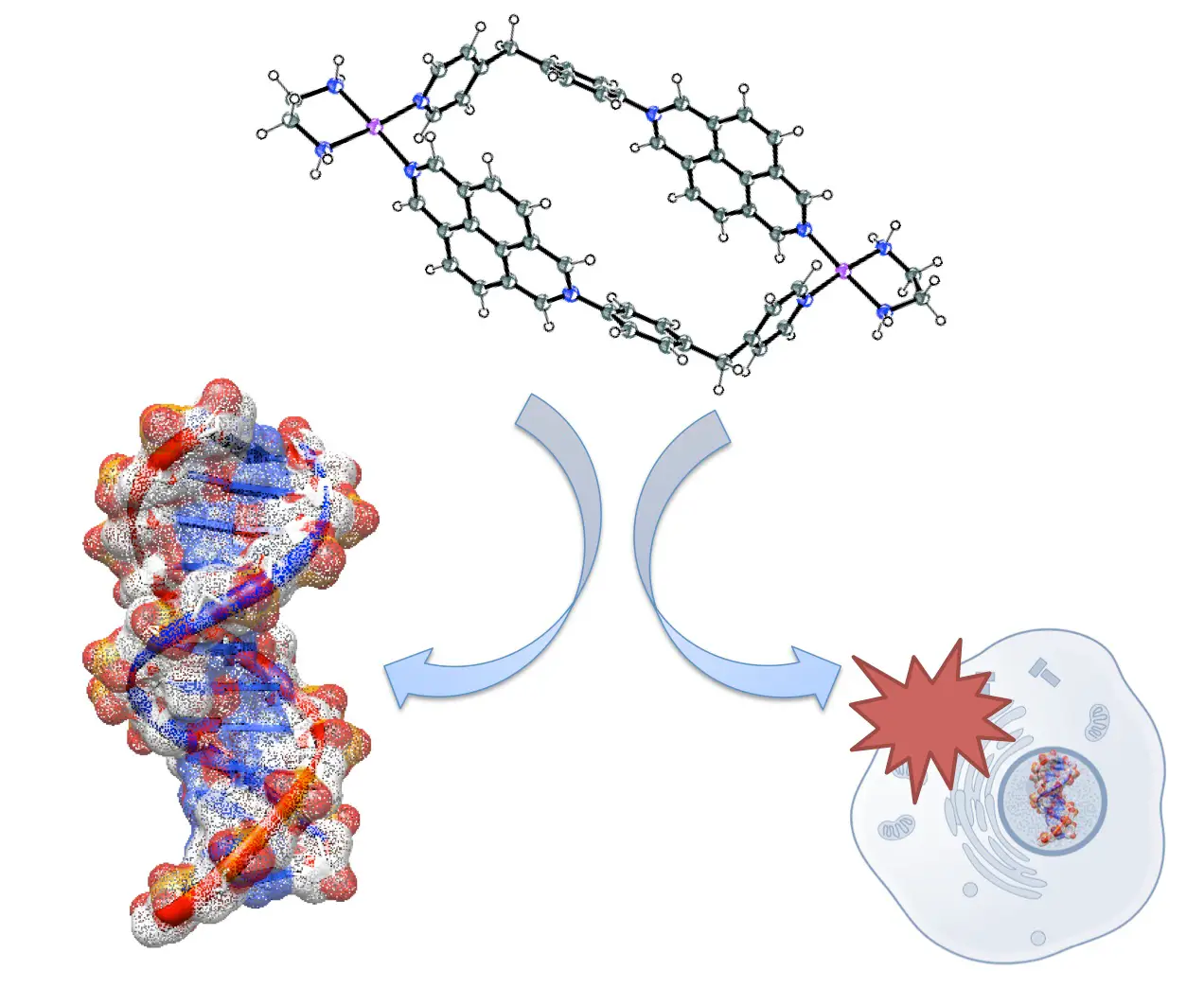
Terenzi, A.; Ducani, C.; Blanco, V.; Zerzankova, L.; Westendorf, A. F.; Peinador, C.; Quintela, J. M.; Bednarski, P. J.; Barone, G.; Hannon, M. J., DNA Binding Studies and Cytotoxicity of a Dinuclear PtII Diazapyrenium-Based Metallo-supramolecular Rectangular Box. Chem. Eur. J. 2012, 18, 10983-10990. DOI: 10.1002/chem.201201519
The interaction with native DNA of a 2,7-diazapyrenium-based ligand 1 and its PtII rectangular metallacycle 2 is explored through circular and linear dichroism and fluorescence spectroscopies. The metal-free ligand 1 binds through intercalation, with a binding constant of approximately 5x105 M-1, whereas the metallacycle 2 binds and bends the DNA with a binding constant of 7x106M-1. PCR assays show that metallo-supramolecular box 2 interferes with DNA transactions in vitro whereas the intercalator 1 does not. The metallacycle is active against four human cancer cell lines, with IC50 values ranging between 3.1 and 19.2 uM and shows similar levels of efficacy, but a different spectrum of activity, to cisplatin.
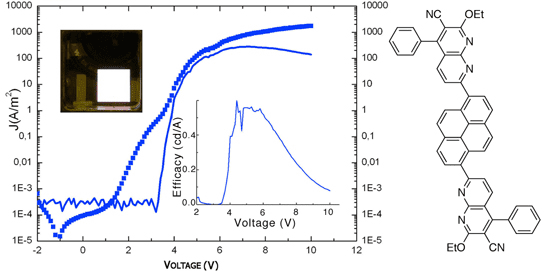
Fernandez-Mato, A.; Quintela, J. M.; Peinador, C., Novel naphthyridine-based compounds in small molecular non-doped OLEDs: synthesis, properties and their versatile applications for organic light-emitting diodes. New J. Chem. 2012, 36, 1634-1640. DOI: 10.1039/c2nj40279c
A series of six new n-type conjugated 1,8-naphthyridine oligomers were examined in organic light emitting diodes (OLEDs). The compounds show a high fluorescence in both solution (10(-8) M) and the solid state. The naphthyridines have high glass-transition (T-g = 65-105 degrees C) and decomposition (T-d = 380-400 degrees C) temperatures, reversible electrochemical reduction, and high electron affinities (2.79-3.00 eV). Systematic variation of the spacer linkage can modulate and fine-tune their properties. They emit high blue, green and yellow photoluminescence with 0.70-1.0 quantum yields. Good-performance, single-layer emitter OLEDs with yellow to white-pink emission have also been demonstrated using these materials. White-pink emitter give a performance with a high brightness (400 cd m(-2) at 4 V), and 0.6 cd A(-1). Yellow emitters give the best performance with maximum brightness of 250 cd m(-2) with a maximum current efficiency of 1.2 cd A(-1). These results demonstrate the potential of this new class of n-type conjugated oligomers as emitters and electron-transport materials for developing high-performance yellow to white-pink OLEDs.
Blanco, V.; Garcia, M. D.; Peinador, C.; Quintela, J. M., Self-assembly of new fluorescent Pd(II) and Pt(II) 2,7-diazapyrenium-based metallocycles and study of their inclusion complexes and 3 catenanes. Chem. Sci. 2011, 2, 2407-2416. DOI: 10.1039/c1sc00508a
New fluorescent square-shaped metallocycles were self-assembled from N-monoalkyl-2,7-diazapyrenium derivatives 1 and 4 and Pd(II)/Pt(II) complexes. Using ligand 1, the metal-directed self-assembly process produced a single metallocycle, while the non-symmetrical salt 4 produced mixtures of regioisomeric Pd(II) and Pt(II) metallocycles upon complexation with the corresponding square-planar cis-complexes. Due to the improved p-deficient character of 1 and 4 compared to related bipyridinium-based ligands, complexation and catenation of the obtained metallocycles with selected electron-rich aromatic substrates produced the corresponding 1 : 2 inclusion complexes and [3]catenanes in a highly efficient fashion. This is particularly relevant for metallocycles derived from ligand 4, as complexation and catenation occurs in a regioselective fashion, generating only the supramolecules with the appropriate parallel arrangement of the diazapyrenium subunits in order to maximize the host-guest pi-stacking interactions. The potential of the new metallocycles for optical signalling applications is illustrated by analyzing their absorption and emission behaviour upon complexation and catenation.
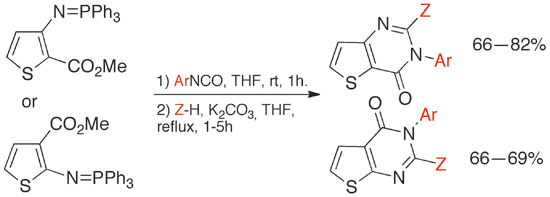
Fernandez-Mato, A.; Peinador, C.; Quintela, J. M., Convenient One-Pot Synthesis of Functionalized Thieno[3,2-d]pyrimidine and Thieno[2,3-d]pyrimidine Derivatives. Synthesis 2011, 3323-3331. DOI: 10.1055/s-0030-1260217
A versatile one-pot method was developed for the preparation of a range of functionalized thieno[3,2-d] pyrimidine and thieno[2,3-d] pyrimidine derivatives. This protocol significantly reduces the cost and time for the synthesis of these compounds in comparison with previous routes, while facilitating scale up to multigram quantities in good yields (66-99%). The technique also permits the synthesis of bis(thienopyrimidine) derivatives not readily available by other methods.
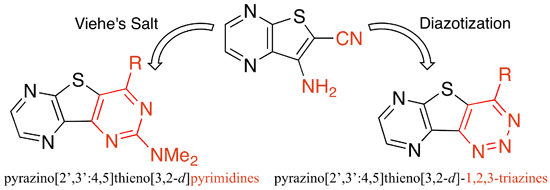
Fernandez-Mato, A.; Peinador, C.; Quintela, J. M., Synthesis and Characterization of Substituted Pyrazino[2’,3’:4,5]thieno[3,2-d]pyrimidines and Related Molecules. Synthesis 2011, 943-953.DOI: 10.1055/s-0030-1258432
An efficient access for the synthesis of substituted pyrazino[2', 3': 4,5] thieno[3,2-d]pyrimidines and pyrazino[ 2', 3': 4,5] thieno[3,2-d]-1,2,3-triazines, heteroaromatic nitrogen ligands, is reported. The title compounds were obtained by functionalization of the corresponding chloro precursors, synthesized by reaction between 7-aminothieno[2,3-b]pyrazine-6-carbonitrile and N,N-dimethyldichloromethyleniminium chloride (Viehe's salt) or sodium nitrite/hydrochloric acid, respectively. Moreover, this procedure provided a convenient synthetic route to bis(pyrazino[2', 3': 4,5]thieno[3,2-d] pyrimidines), functionalized molecules that are not readily available by other synthetic methods.
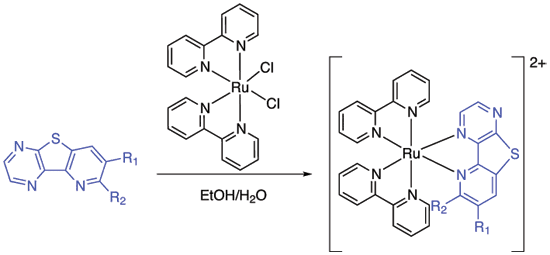
Fernandez-Mato, A.; Quintela, J. M.; Peinador, C.; Platas-Iglesias, C., Preparation and study of pyridothienopyrazines and their Ruthenium(II) complexes: a new family of bidentate ligands. Tetrahedron 2011, 67, 2035-2043. DOI: 10.1016/j.tet.2011.01.066
The Friedlander condensation of 3-aminothieno[2,3-b]pyrazine-2-carboxaldehyde with either methyl ketones or carbocyclic and heterocyclic ketones leads to a family of new bidentate ligands containing a pyridothienopyrazine coordinating unit. Complexation with [Ru(bpy)(2)Cl(2)] affords the corresponding six-coordinated Ru(II) complexes. The structures were analyzed by (1)H NMR spectroscopy, which shows shielding effects reflecting significant interligand pi-stacking interaction in the complexes. The photophysical properties of the ligands and their metallic complexes have been also examined.
Garcia, M. D.; Marti-Rujas, J.; Metrangolo, P.; Peinador, C.; Pilati, T.; Resnati, G.; Terraneo, G.; Ursini, M., Dimensional caging of polyiodides: cation-templated synthesis using bipyridinium salts. Crystengcomm 2011, 13, 4411-4416. DOI: 10.1039/c0ce00860e
The potential of bipyridinium derivatives in the cation templated synthesis of polyiodides has been explored by applying the strategy of size-matching between cations and anions. Bipyridinium cations 1-4, bearing benzyl and functionalized benzyl pendants at nitrogen atoms, are able to template the selective formation of I(4)(2-) and I(3)(-) species. Thanks to the supramolecular space compartmentation induced by the benzyl pendants, the formation of I(4)(2-) and I(3)(-) is independent of the stoichiometry adopted in the crystallization procedure. Bipyridinium cation 5, bearing methyl pendants, is unable to induce space compartmentation and different polyiodides are obtained depending on the stoichiometry used in the crystallization process as the cation-anion size-matching alone does not control the polyiodide formation.